Abstract
Background
Elderly patients with traumatic brain injury increase. Current targets and secondary insult definitions during neurointensive care (NIC) are mostly based on younger patients. The aim was therefore to study the occurrence of predefined secondary insults and the impact on outcome in different ages with particular focus on elderly.
Methods
Patients admitted to Uppsala 2008–2014 were included. Patient characteristics, NIC management, monitoring data, and outcome were analyzed. The percentage of monitoring time for ICP, CPP, MAP, and SBP above-/below-predefined thresholds was calculated.
Results
Five hundred seventy patients were included, 151 elderly ≥ 65 years and 419 younger 16–64 years. Age ≥ 65 had significantly higher percentage of CPP > 100, MAP > 120, and SBP > 180 and age 16–64 had higher percentage of ICP ≥ 20, CPP ≤ 60, and MAP ≤ 80. Age ≥ 65 contributed independently to the different secondary insult patterens. When patients in all ages were analyzed, low percentage of CPP > 100 and SBP > 180, respectively, was significant predictors of favorable outcome and high percentage of ICP ≥ 20, CPP > 100, SBP ≤ 100, and SBP > 180, respectively, was predictors of death. Analysis of age interaction showed that patients ≥ 65 differed and had a higher odds for favorable outcome with large proportion of good monitoring time with SBP > 180.
Conclusions
Elderly ≥ 65 have different patterns of secondary insults/physiological variables, which is independently associated to age. The finding that SBP > 180 increased the odds of favorable outcome in the elderly but decreased the odds in younger patients may indicate that blood pressure should be treated differently depending on age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of neurointensive care (NIC), with focused efforts of avoiding secondary insults, has contributed to an increase of favorable outcome for traumatic brain injury (TBI) patients [2, 3, 8, 23, 27]. Despite this improvement, TBI still constitutes a large health problem. The magnitude of the problem is illustrated by a recent overview of TBI in Europe showing that the incidence of hospitalized TBI patients was 278.2/100 000 in 2012 (Sweden 2013, 451.5/100 000) and the mortality rate was 11.7/100 000 (Sweden 2013, 9.0/100 000) [21]. Despite that elderly (age ≥ 65 years) constituted only 29% of the hospitalized TBI patients, they contributed to 55% of the mortality [21]. It is obvious that the management of elderly TBI patients will be a tremendous challenge for the future for many reasons. In addition to higher mortality rate in the elderly [10, 17, 21], the elderly are an increasing part of the population and they live more active lives than before [10, 17, 18]. Traditionally, there has been some reluctance to treat these patients due to the previous experience of bad outcome, but more recently, larger numbers of elderly are treated [25, 30, 32, 33, 38]. Hence, it is urgent to obtain more knowledge about the optimal treatment of elderly TBI patients.
The NIC of patients with TBI in general is mostly based on data from younger patients and there is insufficient research in the elderly despite the change in population structure [9]. For example, large clinical TBI trials have often been made with age > 65 years as an exclusion criteria [5, 14, 19, 24, 26]. Although the secondary insult prevention concept is one of the main reasons for the improvement of NIC, it is likely that both critical and optimal threshold levels differ between ages. This is underlined by studies in elderly patients with severe subarachnoid hemorrhage showing that the occurrence of defined secondary insults and the impact on outcome was age-dependent [31]. In order to optimize the NIC of elderly TBI patients, it is desirable to identify the critical threshold levels for secondary insults and the optimal threshold levels to target, specifically in the older ages.
The aim of this investigation was therefore to study the occurrence of predefined secondary insults and the impact of outcome in different ages with particular focus on the elderly.
Material and methods
Patient selection and data collection
All TBI patients ≥ 16 years old receiving NIC at Uppsala University Hospital between 2008 and 2014 were retrieved from the Uppsala TBI registry [28]. In total, 663 patients were identified. The following patients were excluded as follows: recovery within 24 h after admission (11 patients), admission more than 5 days after trauma (23 patients), bilateral wide and unresponsive pupils (15 patients) or Glasgow coma scale score 3 and one wide pupil on admission (1 patient) (patients with probable predestined fatal/unfavorable clinical course judged in general not possible to treat [1, 4]), gunshot to head (4) and lost to follow up (39 patients). Finally, 570 patients remained to be analyzed.
Demographics and NIC management data
Demographic data and information about NIC management were obtained from the Uppsala TBI registry [28]. The following parameters were studied as follows: age, sex, primary or secondary transfer, Glasgow coma scale motor score (GCS M) on admission, type of injury, presence of multiple injuries, trauma under the influence of alcohol or drugs, cause of trauma, medical history (brain injury/disease, previous traumatic brain injury, diabetes mellitus, hypertension/cardiovascular disease (CVD), use of anticoagulants/antiplatelets), craniotomy, decompressive craniectomy, intracranial pressure monitoring, and mechanical ventilation. The type of injury was assessed on the initial CT-scan (dominating type of injury and Marshall CT score [22]).
Physiological data
Trended minute-by-minute data (median values of 5 samples during each sampled minute) was collected in real time from the Philips monitors in our ICU using the Odin software [12]. The Philips monitors forward the data to a central database within the hospital, which is queried by the Odin server to extract the relevant data which is stored centrally and displayed on Odin client systems at the ICU bedspaces. The patient data stored and processed by the Odin software is also kept within the hospital firewall. The trended data used in this study were preprocessed with median filters to detect sudden spikes that appeared to be non-physiological, and a specialized algorithm detected sudden drops to a constant value (usually zero). The data were further subject to manual review to verify, and if necessary correct, the automatic procedures. Time gaps from, e.g., radiology examination and surgical procedure were automatically excluded by the Odin software. The monitoring time left was defined as good monitoring time (GMT).
For the purpose of evaluating physiological NIC monitoring data (intra cranial pressure, ICP; cerebral perfusions pressure, CPP; mean arterial pressure, MAP; and systolic blood pressure, SBP), GMT data from the start of monitoring to the end of the seventh monitoring day was studied.For ICP and CPP analyses, at least 12 h of ICP data was required. Using the Odin software, the proportions of good monitoring time (%GMT) spent above-/below-predefined threshold levels were calculated for ICP ≥ 20, CPP ≤ 60, CPP > 100, MAP ≤ 80, MAP > 120, SBP ≤ 100, and SBP > 180. The thresholds originated mainly from our protocol treatment goals [8].
Neurointensive care protocol
All patients were treated according to the same standardized treatment protocol [8]. Unconscious patients (GCS M ≤ 5) had mechanical ventilation. Patients on mechanical ventilation were kept sedated with propofol (Propofol-LipuroB; Braun Medical, Danderyd, Sweden) and received morphine for analgesia. They were initially moderately hyperventilated (PaCO2 4.0–4.5 kPa) with the aim of normoventilation as soon as ICP allowed (ICP < 20 mmHg). Wake-up tests were performed regularly (usually 3–6 times/day unless severe ICP elevations) to assess neurological function. All unconscious patients (GCS M ≤ 5), regardless of age, had also ICP monitoring, except in the case of coagulopathy. An external ventricular drainage system (EVD) (with the pressure dome at the level of the lateral ventricles) was the first choice and an intraparenchymal pressure device was chosen if the ventricles were compressed. Arterial blood pressure was measured with the pressure dome at heart level. Prophylactic anticonvulsants was not used. The treatment goals according to the standardized management protocol were as follows: ICP < 20 mmHg, SBP > 100 mmHg, central venous pressure (CVP) 0–5 mmH2O, CPP > 60 mmHg, blood glucose 5–10 mmol/L, normovolemia, Pa02 > 12 kPa, electrolytes within normal ranges, and body temperature < 38 °C.
Mass lesions in unconscious patients were evacuated.
Raised ICP was treated in a stepwise fashion. If ICP increased ≥ 20 mmHg without mass lesions, cerebrospinal fluid (CSF) was drained from the EVD. Initially small volumes (1–2 ml) were drained intermittently, when there were risk of expanding hematomas and brain swelling. Later CSF was drained using an open system against a pressure level of 15–20 mmHg if needed. If raised ICP persisted, the treatment was escalated with no wake-up tests, continuous sedation with propofol, and stress reduction with ß1-antagonist metoprolol (Seloken®, AstraZeneca AB Södertälje, Sweden) (0.2–0.3 mg/kg/24 h as an infusion) and α2-agonist clonidin (Catapresan®, BoehingerIngelheim AB Stockholm Sweden) (0.5–1.0 μg/kg × 8 or the same dose as an infusion).Thiopental coma treatment and/or decompressive craniectomy were last tier treatment option but were initiated more restrictively in the elderly.
Outcome
The NIC mortality was assessed. Follow-up was done after 6 months, using the extended Glasgow outcome scale (GOSE), by structured telephone interviews done by a few selected persons[34, 39].
Statistics
Differences in the characteristics between age groups were analyzed with Pearsons Chi 2 test.
Mann-Withney U test was used to compare occurrence of secondary insults between the age groups.
Multiple linear regression analysis was done to examine if age ≥ 65 years and admission variables as gender, GCS M, other injuries, extracerebral hematoma, and contusions contributed to the %GMT above/below secondary insult thresholds for the physiological variables.
To evaluate if the %GMT above/below secondary insult thresholds for the physiological variables was associated with outcome, univariate logistic regression analysises were made with favorable outcome (GOSE 5–8) and survival (GOSE 2–8) as dependent variables. To evaluate whether associations differed by age (age 16–64 vs age ≥ 65), multiple logistic regression models were fitted including age, a physiological variable and age by physiological variable interaction as independent variables. The odds ratios (ORs) for physiological variables are reported for each age-group, regardless of the significance of interaction.
p < 0.05 was considered statistically significant. All statistical analyses were carried out in IBM SPSS Statistics for Windows except for Pearsons Chi 2 which was done with Microsoft Excel 365.
Results
Admission characteristics
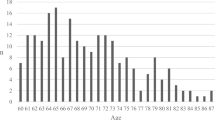
For all patients, the mean age was 49.7 years (range 16–94). The age distribution showed one peak at around 20 years of age and another peak around 60–65 years of age (Supplementary Information 1). There were 151 patients ≥ 65 years (mean 72.3 range 65–87) and 419 between 16 and 64 (mean 41.5 range 16–64) years of age. Patient characteristics from admission are presented in Table 1. When the age groups of ≥ 65 years and 16–64 years were compared, the older patients showed significantly larger proportions of women ( 28.5% vs 19.6%), fall accidents (80.1 vs 42.0%), previous brain injury/disease (22.5% vs 11.0%), diabetes mellitus (18.5 vs 6.2%), hypertension/cerebrovascular disease (58.3% vs 13.8%), ongoing treatment with anticoagulants/antiplatelets (43.0% vs 7.9%), and significantly smaller proportions of patients admitted from other hospitals (67.5% vs 82.3%), multiple injuries (17.9% vs 47.0%), influence of drugs/alcohol (14.6% vs 34.1%), vehicle accidents (7.3% vs 33.2%), and sports injury (0.7% vs 4.3%). Regarding the dominating type of injury assessed on initial CT, the older patients had significantly larger proportion of acute subdural hematoma (51.7% vs 20.5%) and smaller proportion of diffuse axonal injury (DAI) (0.0% vs 8.6%) and epidural hematoma (0.7% vs 11.5%) (Table 2). There was no difference between the age groups in GCS M on admission (Table 1 and Supplementary Information 2).
NIC management and surgery
There were no significant differences between the age groups ≥ 65 years and 16–64 years regarding ICP monitoring (55.0% vs 62.5%) and mechanical ventilation (82.1% vs 77.3%) (Table 3). The proportion of patients treated with thiopental were significantly smaller in the old age group (0.7% vs 7.9%) (Table 3). The old group had significantly more craniotomies compared to the younger group (47.7% vs 32.7%) (Table 3).
Physiological data
Monitoring information regarding number of patients for each physiological parameter and age group is presented in Table 4. When the occurrences of physiological variables were analyzed as median %GMT (Table 5 and Fig. 1), there were statistically significant differences between the age groups: age ≥ 65 years had significantly higher %GMT with CPP > 100, MAP > 120, and SBP > 180 and age 16–64 years had significantly higher %GMT with ICP ≥ 20, CPP ≤ 60, and MAP ≤ 80.
Proportion of good monitoring time (%GMT) for different insult variables by age group. In the box plots, the horizontal black line marks the median, boxes extend from the 25th to the 75th percentile, vertical extending lines denote adjacent values (i.e., the most extreme values within 1.5 interquartile range of the 25th and 75th percentile of each group) and the dots denote observations outside the range of adjacent values (outliers).Mann–Whitney U test, *p < 0.05, **p < 0.01, and ***p < 0.001
The multiple linear regression model with physiological variables as dependent variables and age ≥ 65 years, gender, GCS M, other injuries, extracerebral hematoma, and contusions as explanatory variables showed that age ≥ 65 years was an independent predictor for lower %GMT with ICP ≥ 20 and higher %GMT with CPP > 100, MAP > 120, and SBP > 180 (Table 6). Higher GCS M score was an independent predictor for low %GMT with ICP ≥ 20 and CPP ≤ 60 (Table 6). Other injuries were found to be an independent predictor for lower %GMT with ICP ≥ 20, CPP > 100, MAP > 120, and SBP > 180 and for higher %GMT with MAP ≤ 80 (Table 6). Females showed significanly lower %GMT with SBP > 180 and higher %GMT with SBP ≤ 100. (Table 6).
Outcome
NIC mortality was higher in the old age group (≥ 65 years 8.6% and 16–64 years 2.4%, p < 0.001). Follow-up was made at 7 months in median (range 1–28, including patients who died before follow-up). For all ages, favorable outcome (GOSE 5–8) was observed in 62% (69% in 16–64 years and 42% in elderly) and 13% had died (6% in 16–64 years and 31% in elderly) (Fig. 2).
The results from the logistic regression analyses with favorable outcome and survival as dependent variables and physiological parameters as explanatory variables are presented in Table 7. Low %GMT with CPP > 100 and SBP > 180 were associated with a higher odds of favorable outcome. However, there was a statistically significant interaction between age and %GMT with SBP > 180 (p interaction = 0.025). The OR (per unit increase in %GMT with SBP > 180) was 2.07 (0.22–1731.66) in patients ≥ 65 years and − 0.03(0.00–0.57) in patients 16–64 years (Table 7). High %GMT with ICP ≥ 20, CPP > 100, SBP ≤ 100 were associated with a lower odds of survival (Table 7).
Discussion
Patient and management characteristics by age group
Patients ≥ 65 years of age constituted as much as 26% of all patients. Many of the patient characteristics found in relation to age were as expected. The most common cause of trauma in the elderly was fall accidents, which is in accordance with many other studies [7, 11, 13, 15, 16, 18, 20, 29, 35, 36]. There was a higher percentage of women among the elderly (29% vs 20%), which also was shown by Dams-O’Conner and coll., reporting an increasing proportion of women with increasing age (38.5% in 65–74 years, 50.4% in 75–84 years, and 62.2 in 85 years and older) [7]. The elderly more often had a medical history with previous diseases or injuries, e.g., 22.5% had a previous history of brain injury/disease, 58.3% hypertension/CVD, and 43% medicated with anticoagulants/antiplatelets. Similar results were found by Hawley and coll. showing that older TBI patients ≥ 65 had a recorded medical history in 80% and only 1.1% had no pre-existing medical condition [11]. The dominating injury type in the elderly was ASDH and diffuse injury was also less common according to the Marshall score. These findings are in line with that the dominating type of injury was falls in the elderly and that the elderly more often underwent craniotomy.
Secondary insults/physiological variables—occurrence and association to age
The pattern of secondary insults/physiological variables differed by age. The elderly (≥ 65 years) spent a higher proportion of GMT with high CPP, high MAP, and high SBP and less degree of high ICP, low CPP, and low MAP (Table 5). Similar findings were also observed by Czosnyka and coll. [6].
In order to find out whether the observed difference between the age groups was explained by age independently, a multiple linear regression analysis was performed including age ≥ 65 years as a explanatory factor for the different predefined secondary insults/physiological variables. The analysis showed that age ≥ 65 years was an independent explanatory factor for higher %GMT with CPP > 100, MAP > 120, and SBP > 180 (Table 6). This finding may to some extent be explained by higher degree of hypertension and cardiovascular diseases in the elderly (Table 1). The crucial question is whether higher pressures may influence outcome in a negative way in the elderly.
Secondary insults/physiological variables-relation to clinical outcome and interaction by age
The logistic regression analysis of outcome (favorable and survival) for all patients indicated that high %GMT with ICP > 20, SBP ≤ 100, SBP > 180, CPP > 100 not are beneficial. These findings, which may be summarized roughly as high ICP, low and high BP, and high CPP are bad, were not unexpected. Interestingly, when looking at the interaction analyses, the elderly had a higher AOR for favorable outcome.
Hence, blood pressure should probably be treated differently in younger and older patients. The finding that high blood pressures may be advantageous in elderly is supported by Utomo and coll. who found higher odds of independent living at 6 months for patients ≥ 65 years with a SBP on arrival at hospital in the range of 131–150 mmHg, compared to patients with SBP of < 130 mmHg[37].
ICP did not prove to be a significant predictor of outcome in the elderly. This finding should not be interpreted as if ICP is unimportant for outcome and does not need to be monitored in the elderly. Instead, this is probably an effect of the low burden of ICP insults thanks to effective detection and treatment. We have examined our material for events with %GMT ICP ≥ 25 and there was very few events in the elderly (median %GMT was 0.53, unpublished data). Monitoring of ICP in elderly with TBI is of importance and this has also been shown by You and coll. in a randomized trial of elderly with severe TBI who found lower in-hospital mortality and improved 6-month outcomes for the patients randomized to ICP monitoring [40]. We belive that extensive NIC monitoring is even more important in the elderly due to their increased vulnerability and this philosophy was clearly reflected in the observed numbers of elderly monitored in this study (Table 4), despite a larger proportion elderly using anticoagulants/antiplatelets.
Limitations
This is a single-center study and the results may therefore be influenced by the local management applied. Thus, the results may not be completely generalizable. There was a selection bias since only patients judged to have a reasonable chance for favorable outcome were accepted for NIC. Treatment bias also needs to be considered since all patients were treated to avoid secondary insults and the % GMT at insult level was in low general.
Furthermore, complete multiple logistic regression analyses for assessing the influence of secondary insults on outcome could not be done (to adjust, e.g., sex, GCS at admission, and injury type) due to the relative small number of patients. It was however possible to study the age interaction.
Conclusions
Elderly ≥ 65 years have different patterns of secondary insults/physiological variables, which to some extent is independently associated to age. When patients in all ages were analyzed, low %GMT with CPP > 100 and SBP > 180 were significant predictors of favorable outcome and high %GMT with ICP ≥ 20, CPP > 100, SBP ≤ 100, and SBP > 180 were positive predictors of death. The analysis of age interaction showed that patients ≥ 65 years differed and had a higher odds for favorable outcome and without a significant decrease in survival with large proportion of good monitoring time with SBP > 180.
This finding may indicate that blood pressure should be treated differently in younger and older patients. More TBI studies in the elderly are warrented to define specific guidelines regarding secondary insult definitions and optimal levels to target. Studies of pressure autoregulation and CPPopt are also desirable.
Abbreviations
- ASDH:
-
Acute subdural hematoma
- CPP:
-
Cerebral perfusion pressure
- CPPopt:
-
Optimal cerebral perfusion pressure
- CSF:
-
Cerebrospinal fluid
- CVD:
-
Cardiovascular disease
- CVP:
-
Central venous pressure
- DAI:
-
Diffuse axonal injury
- EDH:
-
Epidural hematoma
- EVD:
-
External ventricular drainage
- GCS M:
-
Glasgow coma scale motor score
- GMT:
-
Good monitoring time
- %GMT:
-
Proportion of good monitoring time
- GOSE:
-
Glasgow outcome scale
- ICP:
-
Intra cranial pressure
- MAP:
-
Mean arterial pressure
- NIC:
-
Neurointensive care
- OR:
-
Odds ratio
- SBP:
-
Systolic blood pressure
- TBI:
-
Traumatic brain injury
References
Andrews BT, Pitts LH (1991) Functional recovery after traumatic transtentorial herniation. Neurosurgery 29(2):227–231
Becker P, Ward JD, Young HF (1977) The outcome from severe head injury with early diagnosis and intensive management. J Neurosurg 4:12
Bowers SA, Marshall LF (1980) Outcome in 200 consecutive cases of severe head injury treated in San Diego County: a prospective analysis. Neurosurgery 6(3):237–242
Combes R, Fauvage B, Jacquot C, Colonna M, Passagia JG, Chirossel JR, de Grenoble C Severe head injuries: an outcome prediction and survival analysis. 5
Cooper DJ, Rosenfeld JV, Murray L et al (2011) Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med 364(16):1493–1502
Czosnyka M, Balestreri M, Steiner L, Smielewski P, Hutchinson PJ, Matta B, Pickard JD (2005) Age, intracranial pressure, autoregulation, and outcome after brain trauma. J Neurosurg 102(3):450–454
Dams-O’Connor K, Cuthbert JP, Whyte J, Corrigan JD, Faul M, Harrison-Felix C (2013) Traumatic brain injury among older adults at level I and II trauma centers. J Neurotrauma 30(24):2001–2013
Elf K, Nilsson P, Enblad P (2002) Outcome after traumatic brain injury improved by an organized secondary insult program and standardized neurointensive care*: Crit Care Med 30(9):2129–2134
Gaastra B, Longworth A, Matta B, Snelson C, Whitehouse T, Murphy N, Veenith T (2016) The ageing population is neglected in research studies of traumatic brain injury. Br J Neurosurg 30(2):221–226
Gardner RC, Dams-O’Connor K, Morrissey MR, Manley GT (2018) Geriatric traumatic brain injury: epidemiology, outcomes, knowledge gaps, and future directions. J Neurotrauma 35(7):889–906
Hawley C, Sakr M, Scapinello S, Salvo J, Wrenn P (2017) Traumatic brain injuries in older adults—6 years of data for one UK trauma centre: retrospective analysis of prospectively collected data. Emerg Med J 34(8):509–516
Howells T, Piper I, Souter M, Miller J (1995) Jones P Design of a research database for the study of secondary insults following head injury. J Neurotrauma 12:471–472
Hukkelhoven CWPM, Steyerberg EW, Rampen AJJ, Farace E, Habbema JDF, Marshall LF, Murray GD, Maas AIR (2003) Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J Neurosurg 99(4):666–673
Hutchinson PJ, Kolias AG, Timofeev IS et al (2016) Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med 375(12):1119–1130
Iaccarino C, Carretta A, Nicolosi F, Morselli C (2018) Epidemiology of severe traumatic brain injury. J Neurosurg Sci 62(5):8
Koskinen S, Alaranta H (2008) Traumatic brain injury in Finland 1991–2005: a nationwide register study of hospitalized and fatal TBI. Brain Inj 22(3):205–214
Lenell S, Nyholm L, Lewén A, Enblad P (2015) Updated periodic evaluation of standardized neurointensive care shows that it is possible to maintain a high level of favorable outcome even with increasing mean age. Acta Neurochir (Wien) 157(3):417–425
Lennartsson C, Heimerson I (2012) Elderly people’s health: Health in Sweden: the National Public Health Report 2012. Chapter 5. Scand J Public Health 40(9 Suppl):95–120
Maas AI, Murray G, Henney H, Kassem N, Legrand V, Mangelus M, Muizelaar J-P, Stocchetti N, Knoller N (2006) Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol 5(1):38–45
Maas AI, Stocchetti N, Bullock R (2008) Moderate and severe traumatic brain injury in adults. Lancet Neurol 7(8):728–741
Majdan M, Plancikova D, Brazinova A, Rusnak M, Nieboer D, Feigin V, Maas A (2016) Epidemiology of traumatic brain injuries in Europe: a cross-sectional analysis. Lancet Public Health 1(2):e76–e83
Marshall LF, Marshall SB, Klauber MR, van Clark M, B, Eisenberg HM, Jane JA, Luerssen TG, Marmarou A, Foulkes MA, (1991) A new classification of head injury based on computerized tomography. J Neurosurg 75(Supplement):S14–S20
Marshall LF, Smith RW, Shapiro HM (1979) The outcome with aggressive treatment in severe head injuries. Part II: acute and chronic barbiturate administration in the management of head injury. J Neurosurg 50(1):26–30
Marshall LF, Maas AIR, Marshall SB et al (1998) A multicenter trial on the efficacy of using tirilazad mesylate in cases of head injury. J Neurosurg 89(4):519–525
Merzo A, Lenell S, Nyholm L, Enblad P, Lewén A (2016) Promising clinical outcome of elderly with TBI after modern neurointensive care. Acta Neurochir (Wien) 158(1):125–133
Nichol A, French C, Little L et al (2015) Erythropoietin in traumatic brain injury (EPO-TBI): a double-blind randomised controlled trial. The Lancet 386(10012):2499–2506
Nordström C-H, Gör Sundbärg, Messeter K, Schalén W (1989) Severe traumatic brain lesions in Sweden. Part 2: impact of aggressive neurosurgical intensive care. Brain Inj 3(3):267–281
Nyholm L, Howells T, Enblad P, Lewén A (2013) Introduction of the Uppsala Traumatic Brain Injury register for regular surveillance of patient characteristics and neurointensive care management including secondary insult quantification and clinical outcome. Ups J Med Sci 118(3):169–180
Peeters W, Majdan M, Brazinova A, Nieboer D, Maas AIR (2017) Changing epidemiological patterns in traumatic brain injury: a longitudinal hospital-based study in Belgium. Neuroepidemiology 48(1–2):63–70
Raj R, Mikkonen ED, Kivisaari R, Skrifvars MB, Korja M, Siironen J (2016) Mortality in elderly patients operated for an acute subdural hematoma: a surgical case series. World Neurosurg 88:592–597
Ryttlefors M, Howells T, Nilsson P, Ronne-Engström E, Enblad P (2007) Secondary insults in subarachnoid hemorrhage: occurrence and impact on outcome and clinical deterioration. Neurosurgery 61(4):704–714; discussion 714–715
Shimoda K, Maeda T, Tado M, Yoshino A, Katayama Y, Bullock MR (2014) Outcome and surgical management for geriatric traumatic brain injury: analysis of 888 cases registered in the Japan Neurotrauma Data Bank. World Neurosurg 82(6):1300–1306
Taussky P, Hidalgo ET, Landolt H, Fandino J (2012) Age and salvageability: analysis of outcome of patients older than 65 years undergoing craniotomy for acute traumatic subdural hematoma. World Neurosurg 78(3–4):306–311
Teasdale GM, Pettigrew LEL, Wilson JTL, Murray G, Jennett B (1998) Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow Outcome Scale. J Neurotrauma 15(8):587–597
Thomas KE, Stevens JA, Sarmiento K (2005) Wald MM (2008) Fall-related traumatic brain injury deaths and hospitalizations among older adults — United States. J Safety Res 39(3):269–272
Thompson HJ, McCormick WC, Kagan SH (2006) Traumatic brain injury in older adults: epidemiology, outcomes, and future implications: traumatic brain injury and older adultS. J Am Geriatr Soc 54(10):1590–1595
Utomo WK, Gabbe BJ, Simpson PM, Cameron PA (2009) Predictors of in-hospital mortality and 6-month functional outcomes in older adults after moderate to severe traumatic brain injury. Injury 40(9):973–977
Wan X, Liu S, Wang S et al (2016) Elderly patients with severe traumatic brain injury could benefit from surgical treatment. World Neurosurg 89:147–152
Wilson JTL, Pettigrew LEL, Teasdale GM (1998) Structured interviews for the Glasgow Outcome Scale and the Extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma 15(8):573–585
You W, Feng J, Tang Q, Cao J, Wang L, Lei J, Mao Q, Gao G, Jiang J (2015) Intraventricular intracranial pressure monitoring improves the outcome of older adults with severe traumatic brain injury: an observational, prospective study. BMC Anesthesiol 16(1):35
Acknowledgements
Mona-Lisa Wernroth at Uppsala Clinical research center, UCR, is acknowledged for statistical expertise advice.
Funding
Open access funding provided by Uppsala University. The study was supported by grants from the Uppsala university hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. The study was approved by the local ethical review board.
Informed consent
Informed consent was obtained from individual participants or the relatives if the participant did not have the decision-making capacity for informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurosurgical intensive care
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lenell, S., Lewén, A., Howells, T. et al. Neurointensive care of traumatic brain injury in the elderly—age-specific secondary insult levels and optimal physiological levels to target need to be defined. Acta Neurochir 164, 117–128 (2022). https://doi.org/10.1007/s00701-021-05047-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-021-05047-z