Abstract
Background
Prevalence, radiological characteristics, and risk factors for peritumoral infarctions after glioma surgery are not much studied. In this study, we assessed shape, volume, and prevalence of peritumoral infarctions and investigated possible associated factors.
Methods
In a prospective single-center cohort study, we included all adult patients operated for diffuse gliomas from January 2007 to December 2018. Postoperative infarctions were segmented using early postoperative MRI images, and volume, shape, and location of postoperative infarctions were assessed. Heatmaps of the distribution of tumors and infarctions were created.
Results
MRIs from 238 (44%) of 539 operations showed restricted diffusion in relation to the operation cavity, interpreted as postoperative infarctions. Of these, 86 (36%) were rim-shaped, 103 (43%) were sector-shaped, 40 (17%) were a combination of rim- and sector-shaped, and six (3%) were remote infarctions. Median infarction volume was 1.7 cm3 (IQR 0.7–4.3, range 0.1–67.1). Infarctions were more common if the tumor was in the temporal lobe, and the map shows more infarctions in the periventricular watershed areas. Sector-shaped infarctions were more often seen in patients with known cerebrovascular disease (47.6% vs. 25.5%, p = 0.024). There was a positive correlation between infarction volume and tumor volume (r = 0.267, p < 0.001) and infarction volume and perioperative bleeding (r = 0.176, p = 0.014). Moreover, there was a significant positive association between age and larger infarction volumes (r = 0.193, p = 0.003). Infarction rates and infarction volumes varied across individual surgeons, p = 0.037 (range 32–72%) and p = 0.026.
Conclusions
In the present study, peritumoral infarctions occurred in 44% after diffuse glioma operations. Infarctions were more common in patients operated for tumors in the temporal lobe but were not more common following recurrent surgeries. Sector-shaped infarctions were more common in patients with known cerebrovascular disease. Increasing age, larger tumors, and more perioperative bleeding were factors associated with infarction volumes. The risk of infarctions and infarction volumes may also be surgeon-dependent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prognosis of diffuse glioma improves with extent of surgical resection [19, 16, 20], but glioma surgery is a balance between extensive tumor resections and avoiding damage to adjacent functional brain tissue. Based on early postoperative magnetic resonance imaging (MRI) with diffusion-weighted imaging (DWI), it has been reported that perioperative and mostly peritumoral infarctions occur in 19–80% of patients undergoing tumor surgery. These infarctions have been associated with postoperative neurological deficits and impaired function [8, 9, 17, 21, 15].
However, characteristics and risk factors for peritumoral infarctions are still not much explored, although some but not all studies report higher risks of infarctions following reoperations [8, 5].
The location of a cerebral infarction is critical for the neurological outcome [13]. In a study of 177 diffuse glioma procedures, it was reported that new postoperative DWI lesions occurred more often in the insula, the operculum, and the temporal lobe [5]. Furthermore, a small study including eleven patients with opercular tumors found that at least nine patients had infarctions in relation to the resection cavity [10]. However, a larger study including 109 patients found no correlation between tumor location and incidence of acquired ischemic lesions, where only a close relation to central arteries was found to be a significant risk factor [8]. Thus, it is still unclear if tumor location in certain brain areas is associated with risk of postoperative ischemic lesions.
In the present study, we sought to assess the shape, volume, and prevalence of peritumoral infarctions, and investigate possible patient- or tumor-related risk factors, including tumor location based on a population-based patient selection and manual volumetric segmentations of postoperative DWI changes.
Methods
Patients and clinical data
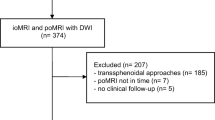
We screened all adult patients (≥ 18 years) operated for newly diagnosed or recurrent diffuse gliomas WHO grades 2–4 at the Department of Neurosurgery at St. Olavs Hospital, Trondheim University Hospital, from January 2007 through December 2018, with available postoperative MRI including DWI performed within 72 h after surgery. This department exclusively serves approximately 750, 000 inhabitants in a defined geographical catchment region.
Patients operated before the second half of 2016 were classified by a neuropathologist according to the 2007 WHO classification of central nervous system-tumors [11], whereas gliomas operated in the latter half of 2016 through 2018 were classified according to the 2016 WHO classification [12].
Clinical data were collected from electronic medical records in a local tumor registry. The patients’ physical status was assessed by an anesthesiologist prior to surgery, using the American Society of Anesthesiologists Classification (ASA). Karnofsky performance status (KPS) was rated by the operating surgeon just prior to surgery, using a questionnaire. Missing clinical data were retrospectively assessed and collected from electrical medical records from all seven hospitals in our catchment region.
MRI scans and DWI analyses
The early postoperative MRI scans consisted of pre-contrast T1, T2, FLAIR, DWI sequences, and a post-contrast T1. DWI was performed according to clinical routine with a standard echo-planar imaging sequence (EPI), and apparent diffusion coefficient (ADC) maps were automatically calculated. Areas with high signals on B1000 images and corresponding low values on ADC maps were considered to have restricted diffusion representing cytotoxic edema following acute ischemia, as long as the anatomical images showed no other explanation, and the relative ADC value (rADC) was lower than 0.7 × 10−3 mm2/s compared to the same area in the contralateral hemisphere. This cutoff value was based on previous studies exploring the time course of ADC map changes in brain ischemia [6]. To exclude diffusion abnormalities related to blood products, areas with high signal on B1000 images that could not clearly be distinguished from areas with high signal on pre-contrast T1 were classified as non-ischemic. Furthermore, preoperative MRI images were also reviewed to exclude other possible sources for the diffusion changes, for instance, likely residual tumor, artifacts, or abscesses.
A medical student trained by an experienced neuroradiologist manually segmented areas with postoperative ischemia using 3D-Slicer version 4.9.0 (3D_Slicer, Boston Massachusetts) based on the areas with high signal on the B1000 changes. In cases of doubt, an experienced neuroradiologist was consulted. Tumors were either semi-automatically or manually segmented from preoperative MRI scans. Non-enhancing or partially enhancing tumors were segmented in 2D or 3D FLAIR volumes, whereas contrast-enhancing tumors were segmented in contrast-enhanced 3D T1 images. Tumor volume segmentations were validated by an experienced neurosurgeon or experienced neuroradiologist.
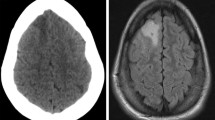
As described in a previous publication from our group [9], DWI abnormalities were classified as either rim (lesions surrounding the cavity), sector, combined (combination of rim and sector), or remote infarctions (not abutting the resection cavity). Minimal areas of increased signal on DWI images in relation to the operation cavity are commonly seen [15, 22]. We therefore used a 3-mm radial diameter cutoff to separate these unspecific postoperative signal changes from radiological significant rim-shaped infarctions (Fig. 1).
Maps of the tumor distribution and infarctions were created based on preoperative tumor segmentations and postoperative infarction segmentations, respectively. All the MR images and corresponding segmentations were spatially aligned with a pre-defined brain template from the Montreal Neurological Institute (MNI—ICBM-152 average brain) [7]. To perform this alignment, the advanced normalization tools framework (ANTs) [1], and more specifically the symmetric diffeomorphic method (SyN), was used. Either the T1 or FLAIR MNI atlas was used to register the preoperative MRI volumes, and the T2 atlas was selected for registering the DWI volumes. A custom and deep learning-based skull-stripping approach has been favored over the built-in approach from the framework. The architecture used is a regular 3D U-Net [23], trained over 300 samples of mixed T1 and FLAIR MRI volumes, and the implementation was done in Python using Keras and Tensorflow. The resulting registration transformations were applied to the individual segmentations to merge all the tumors and infarctions into their common space, yielding the final maps.
Statistical analyses
Statistical analyses were performed with IBS SPSS Statistics version 25.0 (IBM, Armonk, New York). Kolmogorov–Smirnov test and Q-Q-plots were used to determine normal distribution of data. Differences between groups were assessed using independent samples t tests and Pearson’s chi-square tests, for continuous and categorical variables, respectively. Mann–Whitney U and Kruskal–Wallis tests were used for non-parametric data. Spearman’s rank correlation test was used to assess correlation between two continuous variables. Statistical significance level was set to p ≤ 0.05.
Ethics and approval
The study protocol was approved by the Regional Ethical Committee for Health Region Mid-Norway (REK), (REK reference 2018/1187). All patients provided written informed consent (REK reference 2011/974). The data collection was done according to the guidelines of the Helsinki Declaration.
Results
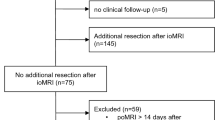
A flowchart of the inclusion process is shown in Fig. 2. Out of 769 eligible operations, 579 gave informed consent to be included in research. Early postoperative MRIs were available following 539 procedures, where 320 were primary operations and 219 were reoperations.
Restricted diffusion in postoperative MRI DWI scans interpreted as infarctions were found in 238 (44%) of the 539 operations, while 301 of 539 (56%) of the postoperative MRI scans had no significant DWI signal changes. Of the 238 infarctions, 86 (36%) were rim-shaped, 103 (43%) were sector-shaped, 40 (17%) were a combination of rim- and sector-shaped, and six (3%) were remote infarctions. In patients with infarctions, the median infarction volume was 1.7 cm3 (inter-quartile range [IQR] 0.7–4.3, range 0.1–67.1). The median infarction volume for sector-shaped or combined rim- and sector-shaped infarctions was significantly larger than operations with rim infarctions alone, 2.4 cm3 vs 1.1 cm3 (p < 0.001). Four out of the six remote infarctions were in patients with glioblastomas, and two were in patients with diffuse low-grade gliomas. Median tumor volume in these six patients was 25.4 ml (IQR 5.6–34.4).
Characteristics of operations with and without postoperative significant DWI signal changes are presented in Table 1. As seen, peritumoral infarctions were more common in patients operated for tumors in the temporal lobe. We did not find any significant associations between postoperative infarctions and WHO grade, tumor lateralization, duration of surgery, ASA grade, preoperative KPS, previous radiotherapy or chemotherapy, or if the surgeon was a resident or consultant. The heatmap presented in Fig. 3 depicts the temporal predominance and shows a possible increased number of infarctions around the horns of the lateral ventricles.
There was a positive correlation between infarction volume and tumor volume (r = 0.267, p < 0.001) and a positive association between infarction volume and intraoperative bleeding (r = 0.176, p = 0.014). Further, there was a significant positive association between age and infarction volumes (r = 0.193, p = 0.003) We found no correlation between infarction volume and the duration of the operations (r = 0.090. p = 0.170).
As a post-hoc analysis, we found a statistically significant difference in infarction rates and infarction volume across individual surgeons, p = 0.037 (range 32–72%) and p = 0.026 (range median 0.3–3.4 cm3), respectively. Subgroup analyses for rim-shaped and sector-shaped infarctions are presented in Tables 2 and 3, respectively. As seen, younger age was associated with more rim-shaped infarctions, while tumors in the temporal lobe and known cerebrovascular disease were associated with sector-shaped infarctions.
Discussion
In this population-based study, we found that peritumoral infarctions as diagnosed by early postoperative DWI are seen following nearly half of the glioma operations. However, most infarctions are small, and median infarction volume in patients with infarctions was only 1.7 ml. Sector-shaped infarctions, or a combination of rim- and sector-shaped infarctions, were larger in volume than rim-shaped infarctions and were seen in approximately one in four patients. Postoperative sector-shaped or combined rim- and sector-shaped infarctions were more common in patients operated for tumors in the temporal lobe and in patients with known cerebrovascular disease. Age and perioperative bleeding were positively associated with larger infarction volumes, while rim-shaped infarctions were more common in younger patients.
Peritumoral infarctions range from very small, rim-like infarctions around the operation cavity to large infarctions that cover a major vascular territory. There is no agreement on the definition of peritumoral infarctions, and the definition used will affect the incidence of such ischemic lesions. Very small DWI abnormalities due to the use of hemostatic agents, small blood clots, and physiological postoperative signal changes are frequently encountered [15, 22]. In the present study, increased DWI signals that measured less than 3 mm in diameter were labeled “not significant”. Although cutoffs may be debated, we earlier found good inter-rater agreement for detecting radiological significant DWI abnormalities when using a pragmatic radiological classification [9].
Still, other studies have classified such minimal rim DWI abnormalities along the surgical cavity as significant, increasing the incidence of infarctions to almost 90% [2]. However, we would argue that such wide definitions are less informative for assessing risk of clinically significant infarctions. Regardless, diffusion restriction should be routinely assessed at the initial postoperative MRI, as the infarctions over time exhibit contrast enhancement, posing a potential challenge in distinguishing infarctions and progressive tumor growth/malignant transformation in subsequent follow-up MRI scans [15].
Previous studies have reached contradictory conclusions as to whether recurrent surgeries are associated with an excess risk of peritumoral infarctions [8, 5]. In a study of 109 operations, peritumoral ischemic lesions were reported in up to 80% of patients after recurrent glioma surgery [8]. In our study, which is the largest to date, we did not find any excess risk of ischemic lesions following reoperations. Thus, fear of peritumoral infarctions should not be a major factor to consider when weighting potential risks of surgery for recurrent glioma.
We found that glioma resections in the temporal lobes were associated with higher risk of ischemic lesions, in line with previous reports [5]. We defined insula as a part of the temporal lobe, and the many perpendicular M3-M4 vessels crossing the insula are a known surgical challenge, which might contribute to the higher risk of ischemic complications and sector-shaped DWI changes in this region. However, another study found no association between the incidence of new ischemic lesions and tumor location [8]. Interestingly, the map-based visualization of infarctions indicates that there are more infarctions around the horns of the lateral ventricles, corresponding to known watershed areas in the brain. The subgroup analysis also revealed a higher rate of sector-shaped infarctions in patients with known cerebrovascular disease, indicating that patient-related vulnerability matters.
While high-grade and low-grade gliomas are very different with respect to (neo)vascularization, it is interesting that the risk of peritumoral infarctions was not associated with histopathology. Thus, the abundance of pathological vessels or edema does not seem to be associated with increased risk of infarctions in normal tissue. Still, increased perioperative bleeding may make it more difficult for the surgeon to separate normal and pathological arteries and ultimately increase the risk of infarctions.
A recent study found an association between persisting neurological deficits and clinically significant DWI changes after resection of diffuse low-grade gliomas [24]. These findings indicated that peritumoral infarctions may be a more common cause of deficits following low-grade glioma surgery than surgical resection of functional brain tissue. Thus, the presence of peritumoral infarctions could have potential as a radiological quality measure in addition to extent of resection following glioma surgery. Studying the clinical impact of peritumoral infarctions is challenging and beyond the scope of this study. Neurological deficits are location-dependent, and infarctions may often occur in areas where loss of function can be difficult to measure. No measured deficits may not be the same as no deficits, as patients may still suffer from less visible or assessable deficits like fatigue, reduced cognitive functions, psychological distress, altered personality, or impaired executive functions. Thus, studying a potential detrimental effect on peritumoral infarctions should perhaps involve neuropsychological testing or measures of health-related quality of life, in addition to conventional neurological deficits and survival [14]. Further research on the long-term effects of peritumoral infarctions on neurological deficits is warranted.
It is still not clear what measures can be taken to minimize the risk of infarctions in relation to tumor surgery. In a study exploring the relationship between perioperative hemodynamics, postoperative infarctions, and overall survival, diastolic blood pressure, a positive liquid balance, and duration of surgery were associated with postoperative infarction volumes [3]. Our data suggest a correlation between perioperative bleeding and infarction volume. However, duration of surgery was not associated with larger infarction volumes or excess risk. This could suggest that both surgical technique and close perioperative anesthesiologic monitoring could be a key to minimize the risks of peritumoral infarctions. The increased risk with age might reflect age-dependent vascular vulnerability due to general vascular disease. The higher rate of sector-shaped infarctions in patients with known cerebrovascular disease and the heatmap showing more infarctions in the watershed areas support that vulnerability of the patients varies.
There are several factors beyond excessive resection of functional brain tissue surrounding the tumor that may cause deficits following surgery, including surgical hematomas, brain infarctions, contusions from spatulas, and postoperative infections. However, the relative importance of these factors is unknown. Several studies emphasize the importance of infarction volume [2,3,4], but even small ischemic lesions in eloquent locations may result in severe neurological sequelae [18]. Further, little is known about the risk of ischemic lesions in relation to surgical technique, e.g., use of suction vs. ultrasonic aspirator, subpial dissection vs. transsulcal approaches, outside-in vs. in-side-out resections, and more. Moreover, it is unknown if the risk of ischemia is surgeon-dependent, or if there is a learning curve. We did not find a significant difference in the incidence of postoperative infarctions between residents and consultant neurosurgeons in the present study. However, in a post-hoc analysis, we found different rates of infarctions and difference in infarction volumes when comparing individual surgeons, indicating that surgical skill or technique may be of importance. However, different surgeons with different degrees of experience and skill operate different tumors, and such comparisons are not necessarily fair. Future studies on the variations of surgical techniques in relation to infarction rates are of interest, but a radiologically and clinical focus on peritumoral infarctions may be of benefit on its own. Experienced glioma surgeons may perhaps remember how implementation of routine, early postoperative MRIs facilitated learning and calibrated their own techniques to obtain better resection grades. Reviewing the DWI sequence carefully after surgery may also be of value to learn and reduce risks in future patients.
The main strengths of the current study are the large sample size, population-based case selection, prospective collection of many data variables, and quantitative analyses based on volumetric segmentation. To the best of our knowledge, this is the largest study reporting the incidence and volumetric segmentations of peritumoral infarctions after glioma surgery. However, false-positive findings are possible in this explorative setting, as we assessed various risk factors for infarctions, without adjusting for multiple testing. Our single-center design could limit the external validity, as surgical treatment for gliomas varies from department to department, both in terms of indications, use of tools, and surgical techniques. Furthermore, infarctions with hemorrhagic transformation may have been falsely scored as no infarctions, as DWI abnormalities which also had hyperintense areas on T1-weighted series were not interpreted as infarctions.
Conclusions
In this population-based cohort study, we found that peritumoral infarctions occurred in 44% after diffuse glioma operations. Infarctions were not more common following recurrent surgeries as compared to primary operations, but infarctions were more common in patients operated for tumors in the temporal lobe. The map-based visualization of infarctions indicates that there are more infarctions around the horns of the lateral ventricles, corresponding to known watershed areas in the brain. Increasing age, larger tumors, and more intraoperative bleeding were factors associated with larger infarction volumes. Infarction rates and infarction volumes differ across individual surgeons.
References
Avants BB, Tustison N, Song G (2009) Advanced normalization tools (ANTS). Insight j 2(365):1–35
Bette S, Wiestler B, Kaesmacher J et al (2016) Infarct volume after glioblastoma surgery as an independent prognostic factor. Oncotarget 7(38):61945–61954. https://doi.org/10.18632/oncotarget.11482 ([publishedOnlineFirst:2016/08/28])
Bette S, Wiestler B, Wiedenmann F et al (2017) Safe brain tumor resection does not depend on surgery alone - role of hemodynamics. Sci Rep 7(1):5585. https://doi.org/10.1038/s41598-017-05767-2 ([publishedOnlineFirst:2017/07/19])
Bette S, Barz M, Huber T et al (2018) Retrospective analysis of radiological recurrence patterns in glioblastoma, their prognostic value and association to postoperative infarct volume. Sci Rep 8(1):4561. https://doi.org/10.1038/s41598-018-22697-9 ([publishedOnlineFirst:2018/03/16])
Dutzmann S, Gessler F, Bink A et al (2012) Risk of ischemia in glioma surgery: comparison of first and repeat procedures. J Neurooncol 107(3):599–607. https://doi.org/10.1007/s11060-011-0784-1 ([publishedOnlineFirst:2012/01/18])
Fiebach JB, Jansen O, Schellinger PD et al (2002) Serial analysis of the apparent diffusion coefficient time course in human stroke. Neuroradiology 44(4):294–298. https://doi.org/10.1007/s00234-001-0720-8 ([publishedOnlineFirst:2002/03/27])
Fonov VS, Evans AC, McKinstry RC et al (2009) Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage 47:S102. https://doi.org/10.1016/S1053-8119(09)70884-5
Gempt J, Forschler A, Buchmann N et al (2013) Postoperative ischemic changes following resection of newly diagnosed and recurrent gliomas and their clinical relevance. J Neurosurg 118(4):801–808. https://doi.org/10.3171/2012.12.JNS12125 ([publishedOnlineFirst:2013/02/05])
Jakola AS, Berntsen EM, Christensen P et al (2014) Surgically acquired deficits and diffusion weighted MRI changes after glioma resection–a matched case-control study with blinded neuroradiological assessment. PLoS ONE 9(7):e101805. https://doi.org/10.1371/journal.pone.0101805 ([publishedOnlineFirst:2014/07/06])
Kumabe T, Higano S, Takahashi S et al (2007) Ischemic complications associated with resection of opercular glioma. J Neurosurg 106(2):263–269. https://doi.org/10.3171/jns.2007.106.2.263 ([publishedOnlineFirst:2007/04/07])
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109. https://doi.org/10.1007/s00401-007-0243-4 ([publishedOnlineFirst:2007/07/10])
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820. https://doi.org/10.1007/s00401-016-1545-1 ([publishedOnlineFirst:2016/05/10])
Menezes NM, Ay H, Wang Zhu M et al (2007) The real estate factor: quantifying the impact of infarct location on stroke severity. Stroke 38(1):194–197. https://doi.org/10.1161/01.STR.0000251792.76080.45[publishedOnlineFirst:2006/11/24]
Sagberg LM, Jakola AS, Solheim O (2014) Quality of life assessed with EQ-5D in patients undergoing glioma surgery: what is the responsiveness and minimal clinically important difference? Qual Life Res 23(5):1427–1434. https://doi.org/10.1007/s11136-013-0593-4 ([publishedOnlineFirst:2013/12/10])
Smith JS, Cha S, Mayo MC et al (2005) Serial diffusion-weighted magnetic resonance imaging in cases of glioma: distinguishing tumor recurrence from postresection injury. J Neurosurg 103(3):428–438. https://doi.org/10.3171/jns.2005.103.3.0428 ([publishedOnlineFirst:2005/10/21])
Smith JS, Chang EF, Lamborn KR et al (2008) Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26(8):1338–1345. https://doi.org/10.1200/JCO.2007.13.9337 ([publishedOnlineFirst:2008/03/08])
Ulmer S, Braga TA, Barker FG 2nd et al (2006) Clinical and radiographic features of peritumoral infarction following resection of glioblastoma. Neurology 67(9):1668–1670. https://doi.org/10.1212/01.wnl.0000242894.21705.3c ([publishedOnlineFirst:2006/11/15])
Wu O, Cloonan L, Mocking SJ et al (2015) Role of acute lesion topography in initial ischemic stroke severity and long-term functional outcomes. Stroke 46(9):2438–2444. https://doi.org/10.1161/STROKEAHA.115.009643 ([publishedOnlineFirst:2015/07/23])
Stummer W, Reulen HJ, Meinel T, et al (2008) Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 62(3):564–76; discussion 64–76. https://doi.org/10.1227/01.neu.0000317304.31579.17 [published Online First: 2008/04/22]
Sanai N, Berger MS (2008) Glioma extent of resection and its impact on patient outcome. Neurosurgery 62(4):753–64; discussion 264–6. https://doi.org/10.1227/01.neu.0000318159.21731.cf [published Online First: 2008/05/23]
Khan RB, Gutin PH, Rai SN, et al (2006) Use of diffusion weighted magnetic resonance imaging in predicting early postoperative outcome of new neurological deficits after brain tumor resection. Neurosurgery 59(1):60–6; discussion 60–6. https://doi.org/10.1227/01.NEU.0000219218.43128.FC [published Online First: 2006/07/11]
Ozturk A, Oguz KK, Akalan N, et al (2006) Evaluation of parenchymal changes at the operation site with early postoperative brain diffusion-weighted magnetic resonance imaging. Diagn Interv Radiol 12(3):115–20. [published Online First: 2006/09/15]
U-net: Convolutional networks for biomedical image segmentation. International Conference on Medical image computing and computer-assisted intervention; 2015. Springer.
Bo HK, Solheim O, Kvistad KA, et al. Intraoperative 3D ultrasound-guided resection of diffuse low-grade gliomas: radiological and clinical results. J Neurosurg 2019:1–12. https://doi.org/10.3171/2018.10.JNS181290 [published Online First: 2019/02/06]
Funding
Open access funding provided by NTNU Norwegian University of Science and Technology (incl St. Olavs Hospital - Trondheim University Hospital).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study protocol was approved by the Regional Ethical Committee for Health Region Mid-Norway (REK) (REK reference 2018/1187).
Patient consent
All patients provided written informed consent (REK reference 2011/974). The data collection was done according to the guidelines of the Helsinki Declaration.
Conflict of interest
All authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Comments
After the introduction of routine MRI after surgery for gliomas, post-operative infarctions in the peritumoral area have become a frequent radiological finding. Nevertheless, there is still little data on this subject in literature. To fill this knowledge gap, the authors conducted a prospective population-based study to investigate the prevalence, radiological characteristics and risk factors of post-operative infarctions.
I commend the authors for this study, finding peritumoral infarctions in almost half (44%) of their cohort while establishing a positive correlation between infarction volume and tumor volume, as well as between infarction volume and perioperative bleeding. Moreover, they also found positive association between larger infarction volumes and patient age. These data constitute important knowledge to surgeons and patients alike; nevertheless, further research in this field elucidating the clinical significance of these findings is warranted.
Jiri Bartek,
Stockholm, Sweden.
This article is part of the Topical Collection on Brain Tumors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Strand, P.S., Berntsen, E.M., Fyllingen, E.H. et al. Brain infarctions after glioma surgery: prevalence, radiological characteristics and risk factors. Acta Neurochir 163, 3097–3108 (2021). https://doi.org/10.1007/s00701-021-04914-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-021-04914-z