Abstract
Background
Butterfly glioblastomas (bGBMs) are grade IV gliomas that infiltrate the corpus callosum and spread to bilateral cerebral hemispheres. Due to the rarity of cases, there is a dearth of information in existing literature. Herein, we evaluate clinical and genetic characteristics, associated predictors, and survival outcomes in an institutional series and compare them to a national cohort.
Methods
We identified all adult patients with bGBM treated at Brigham & Women’s Hospital (2008–2018). The National Cancer Database (NCDB) was also queried for bGBM patients. Survival was analyzed with Kaplan–Meier methods, and Cox models were built to assess for predictive factors.
Results
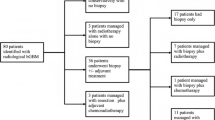
Of 993 glioblastoma patients, 62 cases (6.2%) of bGBM were identified. Craniotomy for resection was attempted in 26 patients (41.9%), with a median volumetric extent of resection (vEOR) of 72.3% (95% confidence interval [95%CI] 58.3–82.1). The IDH1 R132H mutation was detected in two patients (3.2%), and MGMT promoter was methylated in 55.5% of the assessed cases. In multivariable regression, factors predictive of longer OS were increased vEOR, MGMT promoter methylation, and receipt of adjuvant therapy. Median OS for the resected cases was 11.5 months (95%CI 7.7–18.8) vs. 6.3 (95%CI 5.1–8.9) for the biopsied. Of 21,353 GBMs, 719 (3.37%) bGBM patients were identified in the NCDB. Resection was more likely to be pursued in recent years, and GTR was independently associated with prolonged OS (p < 0.01).
Conclusion
Surgical resection followed by adjuvant chemoradiation is associated with significant survival gains and should be pursued in carefully selected bGBM patients.



Similar content being viewed by others
References
Kallenberg K, Goldmann T, Menke J, Strik H, Bock HC, Stockhammer F, Buhk JH, Frahm J, Dechent P, Knauth M (2013) Glioma infiltration of the corpus callosum: early signs detected by DTI. J Neurooncol 112(2):217–222. https://doi.org/10.1007/s11060-013-1049-y
Dziurzynski K, Blas-Boria D, Suki D, Cahill DP, Prabhu SS, Puduvalli V, Levine N (2012) Butterfly glioblastomas: a retrospective review and qualitative assessment of outcomes. J Neurooncol 109(3):555–563. https://doi.org/10.1007/s11060-012-0926-0
Devaux BC, O’Fallon JR, Kelly PJ (1993) Resection, biopsy, and survival in malignant glial neoplasms. A retrospective study of clinical parameters, therapy, and outcome. J Neurosurg 78(5):767–775. https://doi.org/10.3171/jns.1993.78.5.0767
Roche S, Godward S, Middleton A, Lane RJ (1993) Bifrontal glioma presenting as a gross movement disorder. Mov Disord 8(1):120–122. https://doi.org/10.1002/mds.870080124
Steltzer KJ, Sauve KI, Spence AM, Griffin TW, Berger MS (1997) Corpus callosum involvement as a prognostic factor for patients with high-grade astrocytoma. Int J Radiat Oncol Biol Phys 38(1):27–30
Bauman GS, Fisher BJ, Cairncross JG, Macdonald D (1998) Bihemispheric malignant glioma: one size does not fit all. J Neurooncol 38(1):83–89
Hammersen S, Brock M, Cervos-Navarro J (1998) Adult neuronal ceroid lipofuscinosis with clinical findings consistent with a butterfly glioma. Case report. J Neurosurg 88(2):314–318. https://doi.org/10.3171/jns.1998.88.2.0314
Osawa A, Maeshima S, Kubo K, Itakura T (2006) Neuropsychological deficits associated with a tumour in the posterior corpus callosum: a report of two cases. Brain Inj 20(6):673–676. https://doi.org/10.1080/02699050600676958
Balana C, Capellades J, Teixidor P, Roussos I, Ballester R, Cuello M, Arellano A, Florensa R, Rosell R (2007) Clinical course of high-grade glioma patients with a “biopsy-only” surgical approach: a need for individualised treatment. Clin Transl Oncol 9(12):797–803
Agrawal A (2009) Butterfly glioma of the corpus callosum. J Cancer Res Ther 5(1):43–45
Burks JD, Bonney PA, Conner AK, Glenn CA, Briggs RG, Battiste JD, McCoy T, O’Donoghue DL, Wu DH, Sughrue ME (2017) A method for safely resecting anterior butterfly gliomas: the surgical anatomy of the default mode network and the relevance of its preservation. J Neurosurg 126(6):1795–1811. https://doi.org/10.3171/2016.5.Jns153006
Opoku-Darko M, Amuah JE, Kelly JJP (2018) Surgical resection of anterior and posterior butterfly glioblastoma. World Neurosurg 110:e612–e620. https://doi.org/10.1016/j.wneu.2017.11.059
Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG (1999) Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res 59(4):793–797
Kavouridis VK, Boaro A, Dorr J, Cho EY, Iorgulescu JB, Reardon DA, Arnaout O, Smith TR (2019) Contemporary assessment of extent of resection in molecularly defined categories of diffuse low-grade glioma: a volumetric analysis. J Neurosurg: 1–11. https://doi.org/10.3171/2019.6.Jns19972
Boffa DJ, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, Thoburn K, Gress D, McKellar DP, Shulman LN, Facktor MA, Winchester DP (2017) Using the National Cancer Database for outcomes research: a review. JAMA Oncol 3(12):1722–1728. https://doi.org/10.1001/jamaoncol.2016.6905
Harary M, Kavouridis VK, Torre M, Zaidi HA, Chukwueke UN, Reardon DA, Smith TR, Iorgulescu JB (2020) Predictors and early survival outcomes of maximal resection in WHO grade II 1p/19q-codeleted oligodendrogliomas. Neuro Oncol 22(3):369–380. https://doi.org/10.1093/neuonc/noz168
Iorgulescu JB, Torre M, Harary M, Smith TR, Aizer AA, Reardon DA, Barnholtz-Sloan JS, Perry A (2019) The misclassification of diffuse gliomas: rates and outcomes. Clin Cancer Res 25(8):2656–2663. https://doi.org/10.1158/1078-0432.Ccr-18-3101
Chaichana KL, Jusue-Torres I, Lemos AM, Gokaslan A, Cabrera-Aldana EE, Ashary A, Olivi A, Quinones-Hinojosa A (2014) The butterfly effect on glioblastoma: is volumetric extent of resection more effective than biopsy for these tumors? J Neurooncol 120(3):625–634. https://doi.org/10.1007/s11060-014-1597-9
Dayani F, Young JS, Bonte A, Chang EF, Theodosopoulos P, McDermott MW, Berger MS, Aghi MK (2018) Safety and outcomes of resection of butterfly glioblastoma. Neurosurg Focus 44(6):E4. https://doi.org/10.3171/2018.3.Focus1857
Li YM, Suki D, Hess K, Sawaya R (2016) The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg 124(4):977–988. https://doi.org/10.3171/2015.5.Jns142087
Castro BA, Imber BS, Chen R, McDermott MW, Aghi MK (2017) Ventriculoperitoneal shunting for glioblastoma: risk factors, indications, and efficacy. Neurosurgery 80(3):421–430. https://doi.org/10.1227/neu.0000000000001263
Zhu P, Du XL, Blanco AI, Ballester LY, Tandon N, Berger MS, Zhu J-J, Esquenazi Y (2019) Impact of facility type and volume in low-grade glioma outcomes. J Neurosurg:1–11. https://doi.org/10.3171/2019.6.JNS19409
Haque W, Verma V, Butler EB, Teh BS (2017) Definitive chemoradiation at high volume facilities is associated with improved survival in glioblastoma. J Neurooncol 135(1):173–181. https://doi.org/10.1007/s11060-017-2563-0
Hauser A, Dutta SW, Showalter TN, Sheehan JP, Grover S, Trifiletti DM (2018) Impact of academic facility type and volume on post-surgical outcomes following diagnosis of glioblastoma. J Clin Neurosci 47:103–110. https://doi.org/10.1016/j.jocn.2017.10.087
Zhu P, Du XL, Zhu J-J, Esquenazi Y (2019) Improved survival of glioblastoma patients treated at academic and high-volume facilities: a hospital-based study from the National Cancer Database. J Neurosurg:1–12. https://doi.org/10.3171/2018.10.JNS182247
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321(5897):1807–1812. https://doi.org/10.1126/science.1164382
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360(8):765–773. https://doi.org/10.1056/NEJMoa0808710
Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR (2019) Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol 15(7):405–417. https://doi.org/10.1038/s41582-019-0220-2
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352(10):997–1003. https://doi.org/10.1056/NEJMoa043331
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O’Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L (2013) The somatic genomic landscape of glioblastoma. Cell 155(2):462–477. https://doi.org/10.1016/j.cell.2013.09.034
Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tönjes M, Hovestadt V, Albrecht S, Kool M, Nantel A, Konermann C, Lindroth A, Jäger N, Rausch T, Ryzhova M, Korbel JO, Hielscher T, Hauser P, Garami M, Klekner A, Bognar L, Ebinger M, Schuhmann MU, Scheurlen W, Pekrun A, Frühwald MC, Roggendorf W, Kramm C, Dürken M, Atkinson J, Lepage P, Montpetit A, Zakrzewska M, Zakrzewski K, Liberski PP, Dong Z, Siegel P, Kulozik AE, Zapatka M, Guha A, Malkin D, Felsberg J, Reifenberger G, von Deimling A, Ichimura K, Collins VP, Witt H, Milde T, Witt O, Zhang C, Castelo-Branco P, Lichter P, Faury D, Tabori U, Plass C, Majewski J, Pfister SM, Jabado N (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482(7384):226–231. https://doi.org/10.1038/nature10833
Harutyunyan AS, Krug B, Chen H, Papillon-Cavanagh S, Zeinieh M, De Jay N, Deshmukh S, Chen CCL, Belle J, Mikael LG, Marchione DM, Li R, Nikbakht H, Hu B, Cagnone G, Cheung WA, Mohammadnia A, Bechet D, Faury D, McConechy MK, Pathania M, Jain SU, Ellezam B, Weil AG, Montpetit A, Salomoni P, Pastinen T, Lu C, Lewis PW, Garcia BA, Kleinman CL, Jabado N, Majewski J (2019) H3K27M induces defective chromatin spread of PRC2-mediated repressive H3K27me2/me3 and is essential for glioma tumorigenesis. Nat Commun 10(1):1262. https://doi.org/10.1038/s41467-019-09140-x
Meyronet D, Esteban-Mader M, Bonnet C, Joly MO, Uro-Coste E, Amiel-Benouaich A, Forest F, Rousselot-Denis C, Burel-Vandenbos F, Bourg V, Guyotat J, Fenouil T, Jouvet A, Honnorat J, Ducray F (2017) Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol 19(8):1127–1134. https://doi.org/10.1093/neuonc/now274
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Brigham and Women’s Hospital) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study (retrospective), formal consent is not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Comments
The authors present an analysis of 62 butterfly glioblastoma they identified in their institutional records and 719 such lesions identified in a national database. They found extent of resection to be associated with improved survival. Given the retrospective and registry-based nature of the data, laden with confounding by indication, caution should be used when interpreting these results. Nevertheless, one cannot fail to see that a lesion once considered "inoperable" or "not a surgical case" or "showing no benefit after surgery and adjuvant therapy" might still be amenable to surgery in selected cases. As expected, splenial glioblastomas were seldomly resected. The authors have been very strict in their radiological definition of butterfly glioblastoma. It is unclear whether patients in the national database also benefitted from the same strict definition, or whether patients were also included with some corpus callosum infiltration and shift, but no true crossing of the contrast-enhancing component to the contralateral side. Despite the limitations of the data, fact remains that in selected cases surgery for butterfly glioblastoma followed by adjuvant therapy appears to offer benefit in terms of survival, comparable to that of "regular" glioblastoma. Details about patient counselling and which patients to indicate for surgery are needed. These results should prompt in the very least a large-scale international prospective registry effort to gain more insight in the best treatment options for butterfly gliobastoma.
Victor Volovici
Rotterdam, Netherlands
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alessandro Boaro and Vasileios K. Kavouridis shared first authorship.
This article is part of the Topical Collection on Tumor - Glioma
Rights and permissions
About this article
Cite this article
Boaro, A., Kavouridis, V.K., Siddi, F. et al. Improved outcomes associated with maximal extent of resection for butterfly glioblastoma: insights from institutional and national data. Acta Neurochir 163, 1883–1894 (2021). https://doi.org/10.1007/s00701-021-04844-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-021-04844-w