Abstract
Background
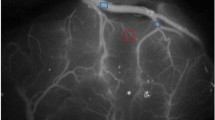
Cerebral hyperperfusion syndrome (CHS) is a common complication after direct bypass surgery in patients with Moyamoya disease (MMD). Since preventive measures may be inadequate, we assessed whether the blood flow difference between the superficial temporal artery (STA) and recipient vessels (△BF) and the direct perfusion range (DPR) are related to CHS.
Methods
We measured blood flow in the STA and recipient blood vessels before bypass surgery by transit-time probe to calculate △BF. Perfusion changes around the anastomosis before and after bypass were analyzed with FLOW800 to obtain DPR. Multiple factors, such as △BF, DPR, and postoperative CHS, were analyzed using binary logistic regression.
Results
Forty-one patients with MMD who underwent direct bypass surgery were included in the study. Postoperative CHS symptoms occurred in 13/41 patients. △BF and DPR significantly differed between the CHS and non-CHS groups. The optimal receiver operating characteristic (ROC) curve cut-off value was 31.4 ml/min for ΔBF, and the area under the ROC curve (AUC) was 0.695 (sensitivity 0.846, specificity 0.500). The optimal cut-off value was 3.5 cm for DPR, and the AUC was 0.702 (sensitivity 0.615, specificity 0.750).
Conclusion
Postoperative CHS is caused by multiple factors. △BF is a risk factor for CHS while DPR is a protective factor against CHS.





Similar content being viewed by others
References
Alaraj A, Ashley WW Jr, Charbel FT, Amin-Hanjani S (2008) The superficial temporal artery trunk as a donor vessel in cerebral revascularization: benefits and pitfalls. Neurosurg Focus 24:E7
Awano T, Sakatani K, Yokose N, Kondo Y, Igarashi T, Hoshino T, Nakamura S, Fujiwara N, Murata Y, Katayama Y, Shikayama T, Miwa M (2010) Intraoperative EC-IC bypass blood flow assessment with indocyanine green angiography in moyamoya and non-moyamoya ischemic stroke. World Neurosurg 73:668–674
Czabanka M, Pena-Tapia P, Schubert GA, Woitzik J, Horn P, Schmiedek P, Vajkoczy P (2009) Clinical implications of cortical microvasculature in adult Moyamoya disease. J Cereb Blood Flow Metab 29:1383–1387
Fujimura M, Mugikura S, Kaneta T, Shimizu H, Tominaga T (2009) Incidence and risk factors for symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease. Surg Neurol 71:442–447
Fujimura M, Niizuma K, Endo H, Sato K, Inoue T, Shimizu H, Tominaga T (2015) Quantitative analysis of early postoperative cerebral blood flow contributes to the prediction and diagnosis of cerebral hyperperfusion syndrome after revascularization surgery for moyamoya disease. Neurol Res 37:131–138
Fujimura M, Shimizu H, Mugikura S, Tominaga T (2009) Delayed intracerebral hemorrhage after superficial temporal artery-middle cerebral artery anastomosis in a patient with moyamoya disease: possible involvement of cerebral hyperperfusion and increased vascular permeability. Surg Neurol 71:223–227 discussion 227
Hamano E, Kataoka H, Morita N, Maruyama D, Satow T, Iihara K, Takahashi JC (2017) Clinical implications of the cortical hyperintensity belt sign in fluid-attenuated inversion recovery images after bypass surgery for moyamoya disease. J Neurosurg 126:1–7
Hwang JW, Yang HM, Lee H, Lee HK, Jeon YT, Kim JE, Lim YJ, Park HP (2013) Predictive factors of symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in adult patients with moyamoya disease. Br J Anaesth 110:773–779
Kim JE, Jeon JS (2014) An update on the diagnosis and treatment of adult Moyamoya disease taking into consideration controversial issues. Neurol Res 36:407–416
Kim JE, Oh CW, Kwon OK, Park SQ, Kim SE, Kim YK (2008) Transient hyperperfusion after superficial temporal artery/middle cerebral artery bypass surgery as a possible cause of postoperative transient neurological deterioration. Cerebrovasc Dis 25:580–586
Kim SJ, Son TO, Kim KH, Jeon P, Hyun SH, Lee KH, Yeon JY, Kim JS, Hong SC, Shin HJ, Bang OY (2014) Neovascularization precedes occlusion in moyamoya disease: angiographic findings in 172 pediatric patients. Eur Neurol 72:299–305
Kim T, Oh CW, Bang JS, Kim JE, Cho WS (2016) Moyamoya disease: treatment and outcomes. J Stroke 18:21–30
Lee M, Guzman R, Bell-Stephens T, Steinberg GK (2011) Intraoperative blood flow analysis of direct revascularization procedures in patients with moyamoya disease. J Cereb Blood Flow Metab 31:262–274
Lundell A, Bergqvist D, Mattsson E, Nilsson B (1993) Volume blood flow measurements with a transit time flowmeter: an in vivo and in vitro variability and validation study. Clin Physiol 13:547–557
Machida T, Nakano S, Ishige S, Ono J, Fujikawa A (2017) Subcortical low-intensity lesions on fluid-attenuated inversion recovery images after revascularization surgery for Moyamoya disease. World Neurosurg 98:512–519
Miyamoto S, Yoshimoto T, Hashimoto N, Okada Y, Tsuji I, Tominaga T, Nakagawara J, Takahashi JC (2014) Investigators JAMT: effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: results of the Japan Adult Moyamoya trial. Stroke 45:1415–1421
Morisawa H, Kawamata T, Kawashima A, Hayashi M, Yamaguchi K, Yoneyama T, Okada Y (2013) Hemodynamics and changes after STA-MCA anastomosis in moyamoya disease and atherosclerotic cerebrovascular disease measured by micro-Doppler ultrasonography. Neurosurg Rev 36:411–419
Nakagawa A, Fujimura M, Arafune T, Sakuma I, Tominaga T (2009) Clinical implications of intraoperative infrared brain surface monitoring during superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease. J Neurosurg 111:1158–1164
Noguchi T, Kawashima M, Nishihara M, Egashira Y, Azama S, Irie H (2015) Noninvasive method for mapping CVR in moyamoya disease using ASL-MRI. Eur J Radiol 84:1137–1143
Ogasawara K, Komoribayashi N, Kobayashi M, Fukuda T, Inoue T, Yamadate K, Ogawa A (2005) Neural damage caused by cerebral hyperperfusion after arterial bypass surgery in a patient with moyamoya disease: case report. Neurosurgery 56:E1380 discussion E1380
Ohue S, Kumon Y, Kohno K, Watanabe H, Iwata S, Ohnishi T (2008) Postoperative temporary neurological deficits in adults with moyamoya disease. Surg Neurol 69:281–286 discussion 286-287
Prinz V, Hecht N, Kato N, Vajkoczy P (2014) FLOW 800 allows visualization of hemodynamic changes after extracranial-to-intracranial bypass surgery but not assessment of quantitative perfusion or flow. Neurosurgery 10(Suppl 2):231–238 discussion 238-239
Research Committee on the Pathology, Treatment of Spontaneous Occlusion of the Circle of Willis, Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases (2012) Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo) 52:245–266
Saito M, Saga T, Hayashi H, Noro S, Wada H, Kamada K (2018) Quantitative blood flow assessment by multiparameter analysis of indocyanine green video angiography. World Neurosurg 116:e187–e193
Suzuki J, Kodama N (1983) Moyamoya disease--a review. Stroke 14:104–109
Uda K, Araki Y, Muraoka S, Ota S, Wada K, Yokoyama K, Nishihori M, Izumi T, Okamoto S, Wakabayashi T (2018) Intraoperative evaluation of local cerebral hemodynamic change by indocyanine green videoangiography: prediction of incidence and duration of postoperative transient neurological events in patients with moyamoya disease. J Neurosurg 1:1–9
Yamaguchi K, Kawamata T, Kawashima A, Hori T, Okada Y (2010) Incidence and predictive factors of cerebral hyperperfusion after extracranial-intracranial bypass for occlusive cerebrovascular diseases. Neurosurgery 67:1548–1554 discussion 1554
Yang T, Higashino Y, Kataoka H, Hamano E, Maruyama D, Iihara K, Takahashi JC (2018) Correlation between reduction in microvascular transit time after superficial temporal artery-middle cerebral artery bypass surgery for moyamoya disease and the development of postoperative hyperperfusion syndrome. J Neurosurg 128:1304–1310
Ye X, Liu XJ, Ma L, Liu LT, Wang WL, Wang S, Cao Y, Zhang D, Wang R, Zhao JZ, Zhao YL (2013) Clinical values of intraoperative indocyanine green fluorescence video angiography with Flow 800 software in cerebrovascular surgery. Chin Med J 126:4232–4237
Author information
Authors and Affiliations
Contributions
Rong Wang and Dongxu Yang conceived and designed the research; Dongxu Yang, Rong Wang, Xiaohong Zhang, Cunxin Tan, Zhiguang Han, Yutao Su, Ran Duan, Guangchao Shi, Junshi Shao, Penghui Cao, and Shihao He performed the research, analyzed the data, and carried out the statistics. Dongxu Yang and Xiaohong Zhang wrote the manuscript, and Rong Wang revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This is a retrospective study and for this type of study formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Comments
The authors perform a retrospective review of 41 patients with MMD who underwent STA-MCA bypass to evaluate for a relationship between changes in pre and post anastomosis blood flow in the donor and recipient vessels as well as FLOW800 are associated with cerebral hyperperfusion syndrome.
The authors do a great job of quantifying a difficult to predict problem.
Fady Charbel
Illinois, USA
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Vascular Neurosurgery
Rights and permissions
About this article
Cite this article
Yang, D., Zhang, X., Tan, C. et al. Intraoperative transit-time ultrasonography combined with FLOW800 predicts the occurrence of cerebral hyperperfusion syndrome after direct revascularization of Moyamoya disease: a preliminary study. Acta Neurochir 163, 563–571 (2021). https://doi.org/10.1007/s00701-020-04599-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04599-w