Abstract
We describe the unusual case of a clinically significant subdural haematoma without any underlying cause in a term baby delivered by an elective caesarean section, which required surgical evacuation. We review the literature and describe the presentation, investigation and management options in infants with this infrequent condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of clinically apparent subdural haematomas in infants is estimated to be 24.1/100,000 [5]. Whilst subdural haematomas may occur due to peripartum trauma, occurrence after elective caesarean section (CS) and requirement for surgical intervention is extremely rare.
Case report
History and examination
A female infant was born at 39 + 3 weeks by elective CS due to previous forceps delivery. Anomaly scan at 20 weeks was normal. There were no complications during pregnancy. Her birth weight was 2860 g and occipital frontal circumference 33 cm (both 9th–25th centiles). She required no resuscitation, had APGAR score of 91 + 95 and normal cord gases. Intramuscular vitamin K was given. She was reviewed at 18 h due to jaundice. Observations were normal, and examination demonstrated normal tone/reflexes and a soft fontanelle. Her total bilirubin was 80 umol/L (below threshold for phototherapy), haemoglobin 178 g/L and platelets 181 × 109/L.
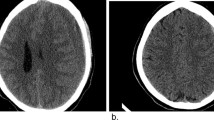
Upon routine newborn examination (30-hours post-delivery), the right pupil was noted to be dilated and non-reactive. The left was normal. The anterior fontanelle was full. There was no abnormality of facial/limb movements, and she was responsive to handling. Ultrasound demonstrated a right subdural haemorrhage (Fig. 1). Repeat bloods showed haemoglobin 182 g/L, platelets 182 × 109/L and a normal coagulation screen. Urgent transfer to the local neurosurgical centre was arranged.
On admission, the only abnormal clinical findings were an isolated right third nerve palsy and a full fontanelle. Under usual circumstances, computed tomography (CT) scan would be performed; however, on this occasion, we had a magnetic resonance imaging (MRI) available immediately. After multidisciplinary discussions, taking into consideration that the baby overall was very well, feeding and stable, we decided on elective intubation and MRI including angiography/venography to assess for possible underlying structural abnormalities (Figs. 2 and 3). No cause was found; however, given the characteristics of the haematoma, a source of bleeding was deemed likely in the occipital inter-hemispheric area. Fontanelle tap of the ventricle to control intracranial pressure (ICP) would likely promote more subdural bleeding. Given the acute nature of the bleed, the haematoma was presumed to be thick/clotted, and we suspected that percutaneous drainage would be ineffective. In theory, conservative management, with further observation, could have been chosen, despite the third nerve palsy, as the baby was otherwise doing well, but risked further bleeding and mass effect, which may have proven fatal. To better control the bleed, mini-craniotomy seemed the best option given the acute nature of the bleed on imaging. The different management strategies were discussed with the parents, and a decision was made to proceed with surgery.
Operation
Under general anaesthesia, in the supine position, a curvilinear incision was made along the ipsilateral coronal suture. Wide draping allowed extension to the occipital area if needed. Periosteum was left intact over the bone and suture. The bone was removed to form a semi-circular craniotomy with its base on the suture. The bone in neonates is very thin and can be cut with scissors and removed using a rongeur/artery clip, or the craniotome can be used for the sake of speed. The bone was pivoted forward, leaving its attachment to the suture intact. The dura was tense, and after opening, surprisingly, dark liquid blood was encountered under pressure resembling subacute/chronic subdural haematoma with some solid clots and then coming later during washout. Further solid clot was encountered occipitally and left in situ as it was the presumed origin of the bleeding. The dura was closed in a watertight fashion and tacked-up to the bone, which was placed back in its native position. The remaining occipital inter-hemispheric haematoma and sealant agent mass were found unchanged on immediate post-operative imaging, as expected.
Post-operative course
The baby made an excellent recovery. There was no discernible neurological deficit, and her right pupil was reacting to light. She was discharged home after 6 days. A repeat MRI (including angiography/venography) was performed 2 months later and showed complete resolution of the subdural haematoma with no parenchymal abnormality, normal vasculature and no underlying cause. Nine months post-operatively, she continues to demonstrate normal development.
Discussion
Subdural haematomas occurring in the peripartum period are almost always caused by the trauma of birth and are usually asymptomatic. Indeed, MRI immediately post-partum demonstrates that 8–46% of infants have asymptomatic subdural collections [12, 20, 25]. Symptomatic subdural haematomas are much more rare, occurring in 16.5/100,000 infants under the age of 1 year [6]. Whilst such cases have been described following emergency/complicated CS, incidence following uncomplicated elective CS is extremely rare [2, 12]. The available evidence is summarized in Table 1.
Presenting symptoms are due to focal neurological deficit or raised ICP. The skull’s elasticity allows some tolerability to increased pressure; therefore, clinical signs may be subtle, such as poor feeding, irritability and frequent vomiting. On examination, scalp veins may be distended and the anterior fontanelle full. Focal neurological deficits may manifest with weakness or cranial nerve deficits. Papilloedema does not usually develop in neonates, but retinal venous engorgement may be noted [19]. If compression of the brainstem occurs, bradycardia and apnoeic spells may manifest.
Ultrasound provides a rapid non-invasive method for diagnosis and can be done at the bedside. CT is also rapid and usually readily available. However, a significant dose of radiation poses long-term implications. MRI does not deliver radiation and provides exquisite images of the brain but can be time-consuming and not as readily available.
It is imperative to assess for potential underlying causes, such as trauma, coagulopathy, meningitis, cerebral venous thrombosis, metabolic diseases or structural lesions (including tumours or vascular malformations) [7,8,9,10,11, 21]. If time permits, MRI is the most helpful imaging tool in ruling out structural causes. Additional sequences that afford an assessment of the cerebral vasculature are vital. This informs best management and likely prognosis, guiding shared decision-making with the patient’s parents/guardians. In this case, a multidisciplinary decision was made to pursue MRI rather than CT in view of these benefits (without significant time penalty), but we emphasize that in most situations, CT remains the imaging of choice for acute intracranial haemorrhage due to its rapidity.
Management of these cases is based upon the patient’s clinical state, the size of the collection and the preference of the surgeon/parents/guardians [15]. A conservative approach could have been considered given her clinical state, but this could have led to sudden rising ICP and deterioration. Subdural transcutaneous puncture or external subdural drainage allows relief of pressure, if the viscosity of the collection permits; however, there is a risk of infection. Neither of these procedures tends to achieve haematoma resolution but can be useful temporizing methods. In a study of 184 children with subdural haematomata in children under 2 years old, Melo et al. [15] found that despite these procedures, 82% and 50% of children, respectively, required further surgery due to recurrent/residual collection. Shunts provide an option to divert the haematoma but again may be ineffective if the clot is thick. Shunting to the subgaleal space may be employed in children under 3 months old as it negates opening the abdomen. Shunting to the peritoneum may be employed in older infants, typically with a valveless system to encourage drainage. The complication rate for shunting in such cases is around 25%, including the risk of recurrent subdural haematoma upon removal, due to tethering of the proximal catheter [15]. Craniotomy achieves immediate access to the subdural space and evacuation of the clot in order to relive associated mass effect. It also allows evaluation of the brain surface to assess for and control any bleeding source. Given the small circulating blood volume in neonates, the risk of blood loss and death are significant.
If an underlying cause for the haemorrhage is demonstrated on imaging, immediate surgical intervention could lead to catastrophic bleeding and prove fatal. The underlying cause may require treatment prior to surgical management, for example, correction of an underlying coagulopathy or aneurysm [7]. Once we had ruled out any underlying cause and decided to perform a craniotomy, the plan was to perform as small a craniotomy as possible and remove the convexity haematoma. We aimed to leave the presumed clot in the posterior inter-hemispheric fissure alone in order not to provoke further bleeding but draped and planned to extend the unicoronal incision posteriorly along the midline in case of uncontrollable bleeding. Fortunately, this was not required; nevertheless, having bailout strategies is vital when operating on neonates.
Long-term outcomes for infants following non-traumatic subdural haematoma are difficult to predict due to a paucity of reported cases with robust follow-up. In cases of non-accidental injury with subdural haematoma, the reported rate of neurological disability is approximately two-thirds [8]. This may be due to the diffuse nature of the brain injury. For subdural haematomas related to accidental trauma, good outcomes are observed in 45–56% of patients [9,10,11]. Vinchon et al. reported sixteen cases of symptomatic spontaneous/atraumatic subdural haematomata, noting that twelve of these had idiopathic macrocrania and seven arachnoidomegaly [24]. They suggest idiopathic macrocephaly/arachnoidomegaly, and dehydration may underlie some cases of spontaneous subdural haematomata. Although not the case in our patient, this study provides a useful comparison in terms of outcome. Over a mean follow-up of 21.2 months, they reported a Glasgow outcome score (GOS) of 1 (normal life) in 87.5%. Outcomes were significantly better compared with a matched cohort of infants sustaining traumatic injuries [24].
In conclusion, clinically apparent subdural haematoma is an unusual condition in the neonatal period. The vast majority of these are due to birth-related trauma, or non-accidental injury, which must be readily suspected and its possibility evaluated on a case-by-case basis. However, spontaneous subdural haematomas exist, and a thorough search for an underlying cause is imperative. These cases are difficult due to uncertainty regarding optimal management strategy. Moreover, there is limited evidence to guide prognosis, and so in these rare cases, close collaboration between the multidisciplinary teams involved, and the patient’s caregivers, is mandatory to guide best treatment for the individual child.
Abbreviations
- CS:
-
Caesarean section
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computed tomography
- ICP:
-
Intracranial pressure
- GOS:
-
Glasgow outcome score
- MRI:
-
Magnetic resonance imaging
- VPS:
-
Ventriculoperitoneal shunt
References
Atluru VL, Kumar IR (1987) Intrauterine chronic subdural haematoma with postoperative tension pneumocephalus. Pediatr Neurol 3:306–309. https://doi.org/10.1016/0887-8994(87)90073-7
Audu L, Mukhtar-yola M, Otunete A, Mairami A, Nwatah V, Mahmud M, Ugwuanyi C, Umar A (2015) Unexplained massive subdural haematoma in a newborn delivered by elective caesarian section: a case report. Niger J Paediatr 43:234–236. https://doi.org/10.4314/njp.v42i3.13
Franklin J, Belkin R, Howieson J, Gallo A (1986) Posterior fossa chronic subdural haematoma in the neonate. AJNR Am J Neuroradiol 7:1099–1100
Gunn TR, Mok PM, Becroft DM (1985) Subdural haemorrhage in utero. Pediatrics 76:605–610
Hobbs C, Childs AM, Wynne J, Livingston J, Seal A (2005) Subdural haematoma and effusion in infancy: an epidemiological study. Arch Dis Child 90:952–955. https://doi.org/10.1136/adc.2003.037739
Hogberg U, Andersson J, Squier W, Hogberg G, Fellman V, Thiblin I, Wester K (2018) Epidemiology of subdural haemorrhage during infancy: a population-based register study. PLoS One 13:e0206340. https://doi.org/10.1371/journal.pone.0206340
Iza-Vallejo B, Mateo-Sierra O, Fortea-Gil F, Ruiz-Juretschke F, Martin YR (2009) Acute subdural haematoma secondary to distal middle cerebral artery aneurysm rupture in a newborn infant. J Neurosurg Pediatr 3:435–438. https://doi.org/10.3171/2009.1.PEDS08176
Jayawant S, Parr J (2007) Outcome following subdural haemorrhages in infancy. Arch Dis Child 92:343–347. https://doi.org/10.1136/adc.2005.084988
Karandikar S, Coles L, Jayawant S, Kemp AM (2004) The neurodevelopmental outcome in infants who have sustained a subdural haemorrhage from non-accidental head injury. Child Abuse Rev 13:178–187. https://doi.org/10.1002/car.850
Keenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF (2004) A population-based comparison of clinical and outcome characteristics of young children with serious inflicted and noninflicted traumatic brain injury. Pediatrics 114:633–639. https://doi.org/10.1542/peds.2003-1020-L
Keenan HT, Runyan DK, Nocera M (2006) Longitudinal follow-up of families and young children with traumatic brain injury. Pediatrics 117:1291–1297. https://doi.org/10.1542/peds.2005-1883
Looney CB, Smith JK, Merck LH, Wolfe HM, Chescheir NC, Hamer RM, Gilmore JH (2007) Intracranial haemorrhage in asymptomatic neonates: prevalence on MR images and relationship to obstetric and neonatal risk factors. Radiology 242:535–541. https://doi.org/10.1148/radiol.2422060133
Ma M, Zhang M, Tang X, Li Z (2017) Massive neonatal intracranial haemorrhage caused by bromadiolone: a case report. Medicine (Baltimore) 96:e8506. https://doi.org/10.1097/MD.0000000000008506
MacDonald JT, Weitz R, Sher PK (1977) Intrauterine chronic subdural haematoma. Arch Neurol 34:777–778. https://doi.org/10.1001/archneur.1977.00500240065012
Melo JR, Di Rocco F, Bourgeois M, Puget S, Blauwblomme T, Sainte-Rose C, Meyer PG, Zerah M (2014) Surgical options for treatment of traumatic subdural haematomas in children younger than 2 years of age. J Neurosurg Pediatr 13:456–461. https://doi.org/10.3171/2014.1.PEDS13393
Menezes AH, Smith DE, Bell WE (1983) Posterior fossa haemorrhage in the term neonate. Neurosurgery 13:452–456. https://doi.org/10.1227/00006123-198310000-00021
Morgan ME, Hensey OJ, Cooke RW (1983) Convexity cerebral haemorrhage in the neonate: in vivo ultrasound diagnosis. Arch Dis Child 58:814–818. https://doi.org/10.1136/adc.58.10.814
Powers CJ, Fuchs HE, George TM (2007) Chronic subdural haematoma of the neonate: report of two cases and literature review. Pediatr Neurosurg 43:25–28. https://doi.org/10.1159/000097521
Rigi M, Almarzouqi SJ, Morgan ML, Lee AG (2015) Papilledema: epidemiology, etiology, and clinical management. Eye Brain 7:47–57. https://doi.org/10.2147/EB.S69174
Rooks VJ, Eaton JP, Ruess L, Petermann GW, Keck-Wherley J, Pedersen RC (2008) Prevalence and evolution of intracranial haemorrhage in asymptomatic term infants. AJNR Am J Neuroradiol 29:1082–1089. https://doi.org/10.3174/ajnr.A1004
Silva AHD, Gander L, Wijesinghe H, Rodrigues D (2019) Spontaneous neonatal subdural haemorrhage: always non-accidental injury? Br J Neurosurg:1–4. https://doi.org/10.1080/02688697.2019.1694134
Tavil B, Korkmaz A, Bayhan T, Aytac S, Unal S, Kuskonmaz B, Yigit S, Cetin M, Yurdakok M, Gumruk F (2016) Foetal and neonatal intracranial haemorrhage in term newborn infants: Hacettepe University experience. Blood Coagul Fibrinolysis 27:163–168. https://doi.org/10.1097/MBC.0000000000000403
Usul H, Karaarslan G, Cakir E, Kuzeyl K, Mungan L, Baykal S (2005) Conservative management of spontaneous posterior fossa subdural haematoma in a neonate. J Clin Neurosci 12:196–198. https://doi.org/10.1016/j.jocn.2004.03.034
Vinchon M, Delestret I, DeFoort-Dhellemmes S, Desurmont M, Noule N (2010) Subdural haematoma in infants: can it occur spontaneously? Data from a prospective series and critical review of the literature. Childs Nerv Syst 26:1195–1205. https://doi.org/10.1007/s00381-010-1105-2
Whitby EH, Griffiths PD, Rutter S, Smith MF, Sprigg A, Ohadike P, Davies NP, Rigby AS, Paley MN (2004) Frequency and natural history of subdural haemorrhages in babies and relation to obstetric factors. Lancet 363:846–851. https://doi.org/10.1016/S0140-6736(04)15730-9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
The participant’s parents have consented to the submission of this case report.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript has not been presented or published previously, either in full or part.
This article is part of the Topical Collection on Pediatric Neurosurgery
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Copley, P.C., Dean, B., Davidson, A.L. et al. Spontaneous subdural haematoma in a neonate requiring urgent surgical evacuation. Acta Neurochir 163, 1743–1749 (2021). https://doi.org/10.1007/s00701-020-04570-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04570-9