Abstract
Background
Recently, several studies have focused on the relationship between blood-brain barrier (BBB) impairment and the etiology of Moyamoya disease (MMD). However, in vivo studies investigating about BBB impairment and cortical perfusion in MMD patients were really rare.
Methods
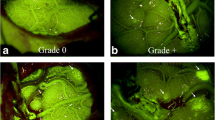
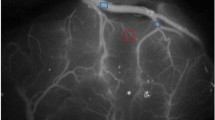
This study included 16 patients diagnosed with MMD and 9 patients with atherosclerotic cerebrovascular disease (ACVD); all of who were treated with superficial temporal artery–middle cerebral artery (STA-MCA) bypass. Cortical perfusion was assessed using intraoperative indocyanine green (ICG) videoangiography by calculating the blood flow index (BFI). In addition, we used sodium fluorescein (NaFl) to evaluate the permeability of BBB in vivo during operation.
Results
The results showed that BBB impairment in MMD patients was more significant than that in ACVD patients, whereas, the cortical perfusion was comparable between two groups. BFI was significantly improved after STA-MCA bypass both in the MMD group (post-operation vs pre-operation: 109.2 ± 67.7 vs 64.3 ± 35.0, p = 0.004) and the ACVD group (post-operation vs pre-operation: 137.6 ± 89.6 vs 90.8 ± 58.3, p = 0.015). Moreover, BFI was significantly decreased in the cortex with BBB impairment as compared with that in the cortex with intact BBB (impaired BBB vs intact BBB: 55.7 ± 26.5 vs 87.6 ± 55.1, p = 0.025). Following bypass, the cortical perfusion significantly improved in the area of BBB impairment (post-operation vs pre-operation: 93.8 ± 75.2 vs 55.7 ± 26.5, p = 0.004), which was not observed in the BBB intact area (post-operation vs pre-operation: 92.4 ± 50.4 vs 87.6 ± 55.1, p = 0.58).
Conclusion
In summary, we observed that BBB impairment in MMD patients was more significant than that in ACVD patients. This study also demonstrated for the first time that cortical perfusion was significantly decreased in the cortex with BBB impairment as compared with that in the cortex with intact BBB in MMD patients. We also observed that After STA-MCA bypass, the cortical perfusion was significantly improved in the cortex with BBB impairment. These results may provide a new insight for BBB impairment and cortical perfusion in the etiology of MMD.




Similar content being viewed by others
References
Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7:41–53. https://doi.org/10.1038/nrn1824
Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV (2010) Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68:409–427. https://doi.org/10.1016/j.neuron.2010.09.043
Blecharz KG, Frey D, Schenkel T, Prinz V, Bedini G, Krug SM, Czabanka M, Wagner J, Fromm M, Bersano A, Vajkoczy P (2017) Autocrine release of angiopoietin-2 mediates cerebrovascular disintegration in Moyamoya disease. J Cereb Blood Flow Metab 37:1527–1539. https://doi.org/10.1177/0271678X16658301
Friedman A (2011) Blood-brain barrier dysfunction, status epilepticus, seizures, and epilepsy: a puzzle of a chicken and egg? Epilepsia 52(Suppl 8):19–20. https://doi.org/10.1111/j.1528-1167.2011.03227.x
Fujimura M, Mugikura S, Kaneta T, Shimizu H, Tominaga T (2009) Incidence and risk factors for symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with moyamoya disease. Surg Neurol 71:442–447. https://doi.org/10.1016/j.surneu.2008.02.031
Fujimura M, Watanabe M, Narisawa A, Shimizu H, Tominaga T (2009) Increased expression of serum matrix metalloproteinase-9 in patients with moyamoya disease. Surg Neurol 72:476–480; discussion 480. https://doi.org/10.1016/j.surneu.2008.10.009
Garrigue P, Giacomino L, Bucci C, Muzio V, Filannino MA, Sabatier F, Dignat-George F, Pisano P, Guillet B (2016) Single photon emission computed tomography imaging of cerebral blood flow, blood-brain barrier disruption, and apoptosis time course after focal cerebral ischemia in rats. Int J Stroke 11:117–126. https://doi.org/10.1177/1747493015607516
Guzman R, Lee M, Achrol A, Bell-Stephens T, Kelly M, Do HM, Marks MP, Steinberg GK (2009) Clinical outcome after 450 revascularization procedures for moyamoya disease. Clinical article. J Neurosurg 111:927–935. https://doi.org/10.3171/2009.4.JNS081649
Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O'Farrell FM, Buchan AM, Lauritzen M, Attwell D (2014) Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508:55–60. https://doi.org/10.1038/nature13165
Hayashi K, Horie N, Suyama K, Nagata I (2012) Incidence and clinical features of symptomatic cerebral hyperperfusion syndrome after vascular reconstruction. World Neurosurg 78:447–454. https://doi.org/10.1016/j.wneu.2011.10.041
Heros RC, Scott RM, Kistler JP, Ackerman RH, Conner ES (1984) Temporary neurological deterioration after extracranial-intracranial bypass. Neurosurgery 15:178–185. https://doi.org/10.1227/00006123-198408000-00006
Huber JD, Egleton RD, Davis TP (2001) Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci 24:719–725. https://doi.org/10.1016/s0166-2236(00)02004-x
Ishihara H, Kubota H, Lindberg RL, Leppert D, Gloor SM, Errede M, Virgintino D, Fontana A, Yonekawa Y, Frei K (2008) Endothelial cell barrier impairment induced by glioblastomas and transforming growth factor beta2 involves matrix metalloproteinases and tight junction proteins. J Neuropathol Exp Neurol 67:435–448. https://doi.org/10.1097/NEN.0b013e31816fd622
Ishii D, Matsushige T, Okazaki T, Shinagawa K, Sakamoto S, Oshita J, Kurisu K (2018) Marked changes in blood-brain barrier biomarkers after direct bypass surgery for Moyamoya angiopathy: preliminary study. World Neurosurg 120:e611–e616. https://doi.org/10.1016/j.wneu.2018.08.134
Kaku Y, Iihara K, Nakajima N, Kataoka H, Fukuda K, Masuoka J, Fukushima K, Iida H, Hashimoto N (2012) Cerebral blood flow and metabolism of hyperperfusion after cerebral revascularization in patients with moyamoya disease. J Cereb Blood Flow Metab 32:2066–2075. https://doi.org/10.1038/jcbfm.2012.110
Kozler P, Pokorny J (2003) Altered blood-brain barrier permeability and its effect on the distribution of Evans blue and sodium fluorescein in the rat brain applied by intracarotid injection. Physiol Res 52:607–614
Kuroda S, Houkin K (2008) Moyamoya disease: current concepts and future perspectives. Lancet Neurol 7:1056–1066. https://doi.org/10.1016/S1474-4422(08)70240-0
Mansour A, Rashad S, Niizuma K, Fujimura M, Tominaga T (2019) A novel model of cerebral hyperperfusion with blood-brain barrier breakdown, white matter injury, and cognitive dysfunction. J Neurosurg:1–13. https://doi.org/10.3171/2019.7.JNS19212
Morisawa H, Kawamata T, Kawashima A, Hayashi M, Yamaguchi K, Yoneyama T, Okada Y (2013) Hemodynamics and changes after STA-MCA anastomosis in moyamoya disease and atherosclerotic cerebrovascular disease measured by micro-Doppler ultrasonography. Neurosurg Rev 36:411–419. https://doi.org/10.1007/s10143-012-0441-y
Nag S, Kapadia A, Stewart DJ (2011) Review: molecular pathogenesis of blood-brain barrier breakdown in acute brain injury. Neuropathol Appl Neurobiol 37:3–23. https://doi.org/10.1111/j.1365-2990.2010.01138.x
Narducci A, Yasuyuki K, Onken J, Blecharz K, Vajkoczy P (2019) In vivo demonstration of blood-brain barrier impairment in Moyamoya disease. Acta Neurochir 161:371–378. https://doi.org/10.1007/s00701-019-03811-w
Roberts TK, Buckner CM, Berman JW (2010) Leukocyte transmigration across the blood-brain barrier: perspectives on neuroAIDS. Front Biosci (Landmark Ed) 15:478–536. https://doi.org/10.2741/3631
Sakamoto S, Kiura Y, Yamasaki F, Shibukawa M, Ohba S, Shrestha P, Sugiyama K, Kurisu K (2008) Expression of vascular endothelial growth factor in dura mater of patients with moyamoya disease. Neurosurg Rev 31:77–81; discussion 81. https://doi.org/10.1007/s10143-007-0102-8
Schoknecht K, Prager O, Vazana U, Kamintsky L, Harhausen D, Zille M, Figge L, Chassidim Y, Schellenberger E, Kovacs R, Heinemann U, Friedman A (2014) Monitoring stroke progression: in vivo imaging of cortical perfusion, blood-brain barrier permeability and cellular damage in the rat photothrombosis model. J Cereb Blood Flow Metab 34:1791–1801. https://doi.org/10.1038/jcbfm.2014.147
Shaik IH, Miah MK, Bickel U, Mehvar R (2013) Effects of short-term portacaval anastomosis on the peripheral and brain disposition of the blood-brain barrier permeability marker sodium fluorescein in rats. Brain Res 1531:84–93. https://doi.org/10.1016/j.brainres.2013.07.040
Soriano SG, Cowan DB, Proctor MR, Scott RM (2002) Levels of soluble adhesion molecules are elevated in the cerebrospinal fluid of children with moyamoya syndrome. Neurosurgery 50:544–549. https://doi.org/10.1097/00006123-200203000-00022
Suzuki J, Takaku A (1969) Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 20:288–299. https://doi.org/10.1001/archneur.1969.00480090076012
Takagi Y, Sawamura K, Hashimoto N, Miyamoto S (2012) Evaluation of serial intraoperative surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography in patients with cerebral arteriovenous malformations. Neurosurgery 70:34–42; discussion 42-33. https://doi.org/10.1227/NEU.0b013e31822d9749
Uchino H, Nakamura T, Houkin K, Murata J, Saito H, Kuroda S (2013) Semiquantitative analysis of indocyanine green videoangiography for cortical perfusion assessment in superficial temporal artery to middle cerebral artery anastomosis. Acta Neurochir 155:599–605. https://doi.org/10.1007/s00701-012-1575-y
Uchino H, Nakayama N, Kazumata K, Kuroda S, Houkin K (2016) Edaravone reduces hyperperfusion-related neurological deficits in adult Moyamoya disease: historical control study. Stroke 47:1930–1932. https://doi.org/10.1161/STROKEAHA.116.013304
Woitzik J, Pena-Tapia PG, Schneider UC, Vajkoczy P, Thome C (2006) Cortical perfusion measurement by indocyanine-green videoangiography in patients undergoing hemicraniectomy for malignant stroke. Stroke 37:1549–1551. https://doi.org/10.1161/01.STR.0000221671.94521.51
Yamaguchi K, Kawamata T, Kawashima A, Hori T, Okada Y (2010) Incidence and predictive factors of cerebral hyperperfusion after extracranial-intracranial bypass for occlusive cerebrovascular diseases. Neurosurgery 67:1548–1554; discussion 1554. https://doi.org/10.1227/NEU.0b013e3181f8c554
Yang Y, Rosenberg GA (2011) Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 42:3323–3328. https://doi.org/10.1161/STROKEAHA.110.608257
Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57:178–201. https://doi.org/10.1016/j.neuron.2008.01.003
Funding
This work was supported by the National Natural Science Foundation of China (No. 81601064).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This protocol was approved by the ethics committee of the First Affiliated Hospital of Soochow University. All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement of informed consent
Informed written consent for this study was obtained for all patients from the patients themselves or their guardians.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Vascular Neurosurgery - Ischemia
Electronic supplementary material
ESM 1
(PDF 71 kb)
Rights and permissions
About this article
Cite this article
Lu, X., Huang, Y., Zhou, P. et al. Decreased cortical perfusion in areas with blood-brain barrier dysfunction in Moyamoya disease. Acta Neurochir 162, 2565–2572 (2020). https://doi.org/10.1007/s00701-020-04480-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04480-w