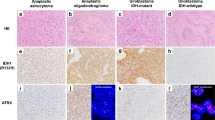
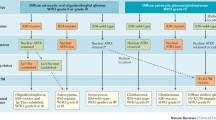
Abstract
The importance of genomic information in intrinsic brain tumors is highlighted in the World Health Organization (WHO) 2016 classification of gliomas, which now incorporates both phenotype and genotype data to assign a diagnosis. By using genetic markers to both categorize tumors and advise patients on prognosis, this classification system has minimized the risk of tissue sampling error, improved diagnostic accuracy, and reduced inter-rater variability. In the neurosurgical community, it is critical to understand the role genetics plays in tumor biology, what certain mutations mean for the patient’s prognosis and adjuvant treatment, and how to interpret the results of sequencing data that are generated following tumor resection. In this review, we examine the critical role of genetics for diagnosis and prognosis and highlight the importance of tumor genetics for neurosurgeons caring for patients with diffuse lower grade gliomas.
Similar content being viewed by others
Abbreviations
- LGG:
-
Low-grade glioma
- GBM:
-
Glioblastoma
- WHO:
-
World Health Organization
- IDH:
-
Isocitrate dehydrogenase
- GWAS:
-
Genome wide association study
- IHC:
-
Immunohistochemistry
- 2-HG:
-
2-Hydroxyglutarate
References
Aghi MK, Nahed BV, Sloan AE, Ryken TC, Kalkanis SN, Olson JJ (2015) The role of surgery in the management of patients with diffuse low grade glioma: a systematic review and evidence-based clinical practice guideline. J Neuro-Oncol 125(3):503–530
Aibaidula A, Chan AKY, Shi Z et al (2017) Adult IDH wild-type lower-grade gliomas should be further stratified. Neuro-Oncology. https://doi.org/10.1093/neuonc/nox078
Andronesi OC, Kim GS, Gerstner E, Batchelor T, Tzika AA, Fantin VR, Vander Heiden MG, Sorensen AG (2012) Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med. https://doi.org/10.1126/scitranslmed.3002693
Baumert BG, Hegi ME, van den Bent MJ et al (2016) Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol 17(11):1521–1532
Beiko J, Suki D, Hess KR et al (2014) IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro-Oncology. https://doi.org/10.1093/neuonc/not159
Bettegowda C, Agrawal N, Jiao Y et al (2011) Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science (80- ). https://doi.org/10.1126/science.1210557
Borger DR, Tanabe KK, Fan KC et al (2012) Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. https://doi.org/10.1634/theoncologist.2011-0386
Broen MPG, Smits M, Wijnenga MMJ, Dubbink HJ, Anten MHME, Schijns OEMG, Beckervordersandforth J, Postma AA, van den Bent MJ (2018) The T2-FLAIR mismatch sign as an imaging marker for non-enhancing IDH-mutant, 1p/19q-intact lower-grade glioma: a validation study. Neuro-Oncology 20(10):1393–1399
Brown TJ, Bota DA, van Den Bent MJ et al (2019) Management of low-grade glioma: a systematic review and meta-analysis. Neurol Pract 6(4):249–258
Buckner JC, Shaw EG, Pugh SL et al (2016) Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. https://doi.org/10.1056/NEJMoa1500925
Bunse L, Pusch S, Bunse T et al (2018) Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med. https://doi.org/10.1038/s41591-018-0095-6
Cairncross G, Wang M, Shaw E et al (2013) Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: Long-term results of RTOG 9402. J Clin Oncol. https://doi.org/10.1200/JCO.2012.43.2674
Cancer Genome Atlas Research Network, Brat DJ, Verhaak RGW et al (2015) Comprehensive, integrative genomic analysis of diffuse lower-gade gliomas. N Engl J Med. https://doi.org/10.1056/NEJMoa1402121
Cancer Genome Atlas Research Network, Brat DJ, Verhaak RGW, Aldape KD, Yung WKA, Salama SR, Cooper LAD et al (2015) Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372(26):2481–2498
Ceccarelli M, Barthel FP, Malta TM et al (2016) Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. https://doi.org/10.1016/j.cell.2015.12.028
Chheda ZS, Kohanbash G, Okada K et al (2018) Novel and shared neoantigen derived from histone 3 variant H3.3K27M mutation for glioma T cell therapy. J Exp Med. https://doi.org/10.1084/jem.20171046
Chittaranjan S, Chan S, Yang C et al (2014) Mutations in CIC and IDH1 cooperatively regulate 2-hydroxyglutarate levels and cell clonogenicity. Oncotarget. https://doi.org/10.18632/oncotarget.2401
Choi C, Ganji SK, DeBerardinis RJ et al (2012) 2-Hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. https://doi.org/10.1038/nm.2682
Clark KH, Villano JL, Nikiforova MN, Hamilton RL, Horbinski C (2013) 1p/19q testing has no significance in the workup of glioblastomas. Neuropathol Appl Neurobiol 39(6):706–717
Coons SW, Johnson PC, Scheithauer BW, Yates AJ, Pearl DK (1997) Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer. https://doi.org/10.1002/(SICI)1097-0142(19970401)79:7<1381::AID-CNCR16>3.0.CO;2-W
Darlix A, Mandonnet E, Freyschlag CF et al (2019) Chemotherapy and diffuse low-grade gliomas: a survey within the European Low-Grade Glioma Network. Neuro-Oncology Pract. https://doi.org/10.1093/nop/npy051
DiCarlo DT, Duffau H, Cagnazzo F, Benedetto N, Morganti R, Perrini P (2018) IDH wild-type WHO grade II diffuse low-grade gliomas. A heterogeneous family with different outcomes. Systematic review and meta-analysis. Neurosurg Rev. https://doi.org/10.1007/s10143-018-0996-3
Dutoit V, Migliorini D, Ranzanici G et al (2018) Antigenic expression and spontaneous immune responses support the use of a selected peptide set from the IMA950 glioblastoma vaccine for immunotherapy of grade II and III glioma. Oncoimmunology. https://doi.org/10.1080/2162402X.2017.1391972
Eckel-Passow JE, Lachance DH, Molinaro AM et al (2015) Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 372(26):2499–2508
Everhard S, Kaloshi G, Criniere E et al (2006) MGMT methylation: a marker of response to temozolomide in low-grade gliomas. Ann Neurol 60(6):740–743
Flavahan WA, Drier Y, Liau BB, Gillespie SM, Venteicher AS, Stemmer-Rachamimov AO, Suvà ML, Bernstein BE (2016) Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature. https://doi.org/10.1038/nature16490
Gilbert MR, Wang M, Aldape KD et al (2013) Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. https://doi.org/10.1200/JCO.2013.49.6968
Glantz MJ, Burger PC, Herndon JE, Friedman AH, Cairncross JG, Vick NA, Schold SC (2012) Influence of the type of surgery on the histologic diagnosis in patients with anaplastic gliomas. Neurology. https://doi.org/10.1212/wnl.41.11.1741
Griffin CA, Burger P, Morsberger L, Yonescu R, Swierczynski S, Weingart JD, Murphy KM (2006) Identification of der(1;19)(q10;p10) in five oligodendrogliomas suggests mechanism of concurrent 1p and 19q loss. J Neuropathol Exp Neurol. https://doi.org/10.1097/01.jnen.0000235122.98052.8f
Haase S, Garcia-Fabiani MB, Carney S, Altshuler D, Núñez FJ, Méndez FM, Núñez F, Lowenstein PR, Castro MG (2018) Mutant ATRX: uncovering a new therapeutic target for glioma. Expert Opin Ther Targets. https://doi.org/10.1080/14728222.2018.1487953
Harary M, Kavouridis VK, Torre M, Zaidi HA, Chukwueke UN, Reardon DA, Smith TR, Iorgulescu JB (2019) Predictors and early survival outcomes of maximal resection in WHO grade II 1p/19q-codeleted oligodendrogliomas. Neuro-Oncology. https://doi.org/10.1093/neuonc/noz168
Hartmann C, Hentschel B, Tatagiba M et al (2011) Molecular markers in low-grade gliomas: predictive or prognostic? Clin Cancer Res 17(13):4588–4599
Hayes J, Yu Y, Jalbert LE et al (2018) Genomic analysis of the origins and evolution of multicentric diffuse lower-grade gliomas. Neuro-Oncology. https://doi.org/10.1093/neuonc/nox205
Hegi ME, Diserens A-C, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352(10):997–1003
Herrlinger U, Tzaridis T, Mack F et al (2019) Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA–09): a randomised, open-label, phase 3 trial. Lancet. https://doi.org/10.1016/S0140-6736(18)31791-4
Hervey-Jumper SL, Berger MS (2016) Maximizing safe resection of low- and high-grade glioma. J Neuro-Oncol. https://doi.org/10.1007/s11060-016-2110-4
Hervey-Jumper SL, van de Bent MJ, Mehta MP, Berger MS (2019) WHO II and III gliomas BT - oncology of cns tumors. In: Tonn J-C, Reardon DA, Rutka JT, Westphal M (eds) . Springer International Publishing, Cham, pp 217–236
Hilf N, Kuttruff-Coqui S, Frenzel K et al (2019) Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 565(7738):240–245
Houillier C, Wang X, Kaloshi G et al (2010) IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology 75(17):1560–1566
Iorgulescu JB, Torre M, Harary M, Smith TR, Aizer AA, Reardon DA, Barnholtz-Sloan JS, Perry A (2019) The misclassification of diffuse gliomas: rates and outcomes. Clin Cancer Res 25(8):2656 LP–2652663
Jenkins RB, Blair H, Ballman KV et al (2006) A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-06-1796
Jenkins RB, Xiao Y, Sicotte H et al (2012) A low-frequency variant at 8q24.21 is strongly associated with risk of oligodendroglial tumors and astrocytomas with IDH1 or IDH2 mutation. Nat Genet. https://doi.org/10.1038/ng.2388
Kannan K, Inagaki A, Silber J, Gorovets D, Zhang J, Kastenhuber ER, Heguy A, Petrini JH, Chan TA, Huse JT (2012) Whole exome sequencing identifies ATRX mutation as a key molecular determinant in lower-grade glioma. Oncotarget. https://doi.org/10.18632/oncotarget.689
Karim ABMF, Maat B, Hatlevoll R et al (1996) A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European organization for research and treatment of cancer (EORTC) study 22844. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/S0360-3016(96)00352-5
Kavouridis VK, Boaro A, Dorr J, Cho EY, Iorgulescu JB, Reardon DA, Arnaout O, Smith TR (2019) Contemporary assessment of extent of resection in molecularly defined categories of diffuse low-grade glioma: a volumetric analysis. J Neurosurg 1(aop):1–11
Khayal IS, Nelson SJ (2009) Characterization of low-grade gliomas using RGB color maps derived from ADC histograms. J Magn Reson Imaging. https://doi.org/10.1002/jmri.21810
Khayal IS, Vandenberg SR, Smith KJ, Cloyd CP, Chang SM, Cha S, Nelson SJ, McKnight TR (2011) MRI apparent diffusion coefficient reflects histopathologic subtype, axonal disruption, and tumor fraction in diffuse-type grade II gliomas. Neuro-Oncology. https://doi.org/10.1093/neuonc/nor122
Killela PJ, Reitman ZJ, Jiao Y et al (2013) TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.1303607110
Kim BYS, Jiang W, Beiko J, Prabhu SS, Demonte F, Gilbert MR, Sawaya R, Aldape KD, Cahill DP, McCutcheon IE (2014) Diagnostic discrepancies in malignant astrocytoma due to limited small pathological tumor sample can be overcome by IDH1 testing. J Neuro-Oncol. https://doi.org/10.1007/s11060-014-1451-0
Kinnersley B, Labussière M, Holroyd A et al (2015) Genome-wide association study identifies multiple susceptibility loci for glioma. Nat Commun. https://doi.org/10.1038/ncomms9559
Leu S, von Felten S, Frank S et al (2013) IDH/MGMT-driven molecular classification of low-grade glioma is a strong predictor for long-term survival. Neuro-Oncology 15(4):469–479
Levin N, Lavon I, Zelikovitsh B, Fuchs D, Bokstein F, Fellig Y, Siegal T (2006) Progressive low-grade oligodendrogliomas: response to temozolomide and correlation between genetic profile and O6-methylguanine DNA methyltransferase protein expression. Cancer 106(8):1759–1765
Li H, Li J, Cheng G, Zhang J, Li X (2016) IDH mutation and MGMT promoter methylation are associated with the pseudoprogression and improved prognosis of glioblastoma multiforme patients who have undergone concurrent and adjuvant temozolomide-based chemoradiotherapy. Clin Neurol Neurosurg. https://doi.org/10.1016/j.clineuro.2016.10.004
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. https://doi.org/10.1007/s00401-016-1545-1
Lu Y, Kwintkiewicz J, Liu Y et al (2017) Chemosensitivity of IDH1-mutated gliomas due to an impairment in PARP1-mediated DNA repair. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-16-2773
Malmer B, Grönberg H, Bergenheim AT, Lenner P, Henriksson R (1999) Familial aggregation of astrocytoma in Northern Sweden: an epidemiological cohort study. Int J Cancer. https://doi.org/10.1002/(SICI)1097-0215(19990505)81:3<366::AID-IJC9>3.0.CO;2-0
Mardis ER, Ding L, Dooling DJ et al (2009) Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. https://doi.org/10.1056/NEJMoa0903840
McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A (2009) Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. https://doi.org/10.1227/01.NEU.0000349763.42238.E9
Mellinghoff IK, Touat M, Maher E et al (2017) ACTR-46. AG-120, A first-in-class mutant idh1 inhibitor in patients with recurrent or progressive idh1 mutant glioma: updated results from the phase 1 non-enhancing glioma population. Neuro-Oncology. https://doi.org/10.1093/neuonc/nox168.037
Mellinghoff IK, Penas-Prado M, Peters KB et al (2018) Phase 1 study of AG-881, an inhibitor of mutant IDH1/IDH2, in patients with advanced IDH-mutant solid tumors, including glioma. J Clin Oncol 36(15_suppl):2002
Nitta M, Muragaki Y, Maruyama T et al (2015) Proposed therapeutic strategy for adult low-grade glioma based on aggressive tumor resection. Neurosurg Focus. https://doi.org/10.3171/2014.10.FOCUS14651
Noushmehr H, Weisenberger DJ, Diefes K et al (2010) Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. https://doi.org/10.1016/j.ccr.2010.03.017
Ochs K, Ott M, Bunse T et al (2017) K27M-mutant histone-3 as a novel target for glioma immunotherapy. Oncoimmunology. https://doi.org/10.1080/2162402X.2017.1328340
Ohgaki H, Kleihues P (2013) The definition of primary and secondary glioblastoma. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-12-3002
Olar A, Wani KM, Alfaro-Munoz KD et al (2015) IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II–III diffuse gliomas. Acta Neuropathol. https://doi.org/10.1007/s00401-015-1398-z
Osswald M, Jung E, Sahm F et al (2015) Brain tumour cells interconnect to a functional and resistant network. Nature. https://doi.org/10.1038/nature16071
Osswald M, Solecki G, Wick W, Winkler F (2016) A malignant cellular network in gliomas: potential clinical implications. Neuro-Oncology. https://doi.org/10.1093/neuonc/now014
Patel T, Bander ED, Venn RA et al (2017) The role of extent of resection in IDH1 wild-type or mutant low-grade gliomas. Neurosurgery. https://doi.org/10.1093/neuros/nyx265
Patel SH, Poisson LM, Brat DJ et al (2017) T2–FLAIR mismatch, an imaging biomarker for IDH and 1p/19q status in lower-grade gliomas: a TCGA/TCIA project. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-17-0560
Platten M, Bunse L, Riehl D, Bunse T, Ochs K, Wick W (2018) Vaccine strategies in gliomas. Curr Treat Options Neurol. https://doi.org/10.1007/s11940-018-0498-1
Pope WB, Prins RM, Thomas MA et al (2012) Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J Neuro-Oncol. https://doi.org/10.1007/s11060-011-0737-8
Rajaraman P, Melin BS, Wang Z et al (2012) Genome-wide association study of glioma and meta-analysis. Hum Genet. https://doi.org/10.1007/s00439-012-1212-0
Reifenberger J, Reifenberger G, Liu L, James CD, Wechsler W, Collins VP (1994) Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p. Am J Pathol
Reuss DE, Mamatjan Y, Schrimpf D et al (2015) IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol. https://doi.org/10.1007/s00401-015-1438-8
Rice T, Lachance DH, Molinaro AM et al (2015) Understanding inherited genetic risk of adult glioma – a review. Neuro-Oncol Pract. https://doi.org/10.1093/nop/npv026
Sahm F, Koelsche C, Meyer J, Pusch S, Lindenberg K, Mueller W, Herold-Mende C, Von Deimling A, Hartmann C (2012) CIC and FUBP1 mutations in oligodendrogliomas, oligoastrocytomas and astrocytomas. Acta Neuropathol. https://doi.org/10.1007/s00401-012-0993-5
Sahm F, Reuss D, Koelsche C et al (2014) Farewell to oligoastrocytoma: in situ molecular genetics favor classification as either oligodendroglioma or astrocytoma. Acta Neuropathol. https://doi.org/10.1007/s00401-014-1326-7
Sanson M, Hosking FJ, Shete S et al (2011) Chromosome 7p11.2 (EGFR) variation influences glioma risk. Hum Mol Genet. https://doi.org/10.1093/hmg/ddr192
Schiff D, Soffietti R, Huse J, Lawler S, Hegi M (2017) The clinical value of ATRX and TERT mutations in diffuse adult gliomas. Neuro-Oncology. https://doi.org/10.1093/neuonc/nox185
Schumacher T, Bunse L, Pusch S et al (2014) A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. https://doi.org/10.1038/nature13387
Schwartzentruber J, Korshunov A, Liu XY et al (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. https://doi.org/10.1038/nature10833
Shete S, Hosking FJ, Robertson LB et al (2009) Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet. https://doi.org/10.1038/ng.407
Shirahata M, Ono T, Stichel D et al (2018) Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. https://doi.org/10.1007/s00401-018-1849-4
Stacey SN, Sulem P, Jonasdottir A et al (2011) A germline variant in the TP53 polyadenylation signal confers cancer susceptibility. Nat Genet. https://doi.org/10.1038/ng.926
Sulkowski PL, Corso CD, Robinson ND et al (2017) 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aal2463
Suzuki H, Aoki K, Chiba K et al (2015) Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet. https://doi.org/10.1038/ng.3273
Turcan S, Rohle D, Goenka A et al (2012) IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. https://doi.org/10.1038/nature10866
VanDenBent MJ (2010) Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician’s perspective. Acta Neuropathol. https://doi.org/10.1007/s00401-010-0725-7
VanDenBent MJ, Brandes AA, Taphoorn MJB et al (2013) Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: Long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. https://doi.org/10.1200/JCO.2012.43.2229
VanDenBent MJ, Hartmann C, Preusser M et al (2013) Interlaboratory comparison of IDH mutation detection. J Neuro-Oncol. https://doi.org/10.1007/s11060-013-1056-z
Ward PS, Patel J, Wise DR et al (2010) The Common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting α-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. https://doi.org/10.1016/j.ccr.2010.01.020
Wiestler B, Capper D, Holland-Letz T, Korshunov A, Von Deimling A, Pfister SM, Platten M, Weller M, Wick W (2013) ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta Neuropathol. https://doi.org/10.1007/s00401-013-1156-z
Wijnenga MMJ, Dubbink HJ, French PJ, Synhaeve NE, Dinjens WNM, Atmodimedjo PN, Kros JM, Dirven CMF, Vincent AJPE, van den Bent MJ (2017) Molecular and clinical heterogeneity of adult diffuse low-grade IDH wild-type gliomas: assessment of TERT promoter mutation and chromosome 7 and 10 copy number status allows superior prognostic stratification. Acta Neuropathol 134(6):957–959
Wrensch M, Lee M, Miike R, Newman B, Barger G, Davis R, Wiencke J, Neuhaus J (1997) Familial and personal medical history of cancer and nervous system conditions among adults with glioma and controls. Am J Epidemiol. https://doi.org/10.1093/oxfordjournals.aje.a009154
Wrensch M, Jenkins RB, Chang JS et al (2009) Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet. https://doi.org/10.1038/ng.408
Wu G, Broniscer A, McEachron TA et al (2012) Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. https://doi.org/10.1038/ng.1102
Yan H, Parsons DW, Jin G et al (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med. https://doi.org/10.1056/NEJMoa0808710
You H, Wu Y, Chang K, Shi X, Chen X-D, Yan W, Li R (2017) Paradoxical prognostic impact of TERT promoter mutations in gliomas depends on different histological and genetic backgrounds. CNS Neurosci Ther 23(10):790–797
Young JS, Prados MD, Butowski N (2018) Using genomics to guide treatment for glioblastoma. Pharmacogenomics. https://doi.org/10.2217/pgs-2018-0078
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Comments
This valuable review deals with the current molecular and histological classification of low-grade gliomas and addresses the therapeutic and prognostic implications of such recent acquisitions. References are appropriate and the timeline highlighted in Table 1, of help in summarizing the pivotal discoveries of this field. Since Bailey and Cushing’s proposal in 1926, human gliomas have been classified according to their histological features, lineage of differentiation, and putative embryological origin. In the course of the twentieth century, Bailey and Cushing’s model inspired huge achievements on glioma biology, leading to a progressive refinement of these tumors’ classification.
In recent years, the understanding of the molecular events driving gliomagenesis set the bases for novel integrated definitions of each tumor entity. This approach is endorsed by the current WHO classification of central nervous system tumors, which (for the first time in the history of glioma classification) identifies both pathological and molecular criteria for their diagnosis. Yet, for sure, this is not the end of a century long medical adventure.
Since the publication of the 2016 WHO classification, many steps forward have been taken, which will likely change the landscape of glioma diagnosis, therapy, and prognostic stratification. These novel acquisitions include (i) tumor epigenetic and methylation profiles, (ii) single cell resolution of molecular and metabolic features, (iii) the characterization of intra-tumor clonal heterogeneity, and (iv) an in-depth definition of the tumor microenvironment. These studies highlight a much more complex scenario than previously thought and pose challenging questions to the current neuro-oncology practice.
Only a tight collaboration among health professionals will succeed in the management of these complex diseases.
Domenico d’Avella and Marco Pizzi Padova, Italy
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Tumor - Glioma
Rights and permissions
About this article
Cite this article
Young, J.S., Gogos, A.J., Morshed, R.A. et al. Molecular characteristics of diffuse lower grade gliomas: what neurosurgeons need to know. Acta Neurochir 162, 1929–1939 (2020). https://doi.org/10.1007/s00701-020-04426-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04426-2