Abstract
Background
Pressure reactivity index (PRx) has emerged as a means to continuously monitor cerebrovascular reactivity in traumatic brain injury (TBI). However, other intracranial pressure (ICP)-based continuous metrics exist, and may have advantages over PRx. The goal of this study was to perform a scoping overview of the literature on non-PRx ICP-based continuous cerebrovascular reactivity metrics in adult TBI.
Methods
We searched MEDLINE, BIOSIS, EMBASE, Global Health, SCOPUS, and Cochrane Library from inception to December 2019. Using a two-stage filtering of title/abstract, and then full manuscript, we identified pertinent articles. Data was abstracted to tables and each technique summarized, including pulse amplitude index (PAx), correlation between pulse amplitude of ICP and cerebral perfusion pressure (RAC), PRx55-15, and low-resolution metrics LAx and L-PRx.
Results
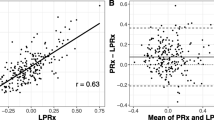
A total of 23 articles met the inclusion criteria, with the vast majority being retrospective in nature and based out of European centers. Sixteen articles focused on high-resolution metrics PAx, RAC, and PRx55-15, with 6 articles focusing on LAx and L-PRx. PAx may have a role in low ICP situations, where it appears to perform superior to PRx. RAC displays similar behavior to PRx, with a trend to stronger associations with favorable/unfavorable outcome at 6 months, and stronger parabolic relationship with CPP. PRx55-15 provides a focused assessment on the vasogenic frequency range associated with cerebral autoregulation, with preliminary data supporting a strong association with outcome in TBI. LAx and L-PRx display varying associations with 6-month outcome in TBI, depending on the window length of calculation, with shorter windows demonstrating stronger correlations with classical PRx.
Conclusions
Non-PRx continuous ICP-based cerebrovascular reactivity metrics can be split into high-resolution and low-resolution measures. High-resolution indices include PAx, RAC, and PRx55-15, while low-resolution indices include L-PRx and LAx. The true role for these metrics beyond classic PRx remains unclear. Each displays situations where it may prove superior over PRx, given limitations with this currently widely accepted measure. Much future investigation into each of these alternative metrics is required prior to adoption into the clinical monitoring armamentarium in adult TBI.
Similar content being viewed by others
References
Andresen M, Donnelly J, Aries M, Juhler M, Menon D, Hutchinson P, Smielewski P (2018) Further controversies about brain tissue oxygenation pressure-reactivity after traumatic brain injury. Neurocrit Care 28(2):162–168
Aries MJH, Czosnyka M, Budohoski KP et al (2012) Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med 40(8):2456–2463
Aries MJH, Czosnyka M, Budohoski KP, Kolias AG, Radolovich DK, Lavinio A, Pickard JD, Smielewski P (2012) Continuous monitoring of cerebrovascular reactivity using pulse waveform of intracranial pressure. Neurocrit Care 17(1):67–76
Beqiri E, Smielewski P, Robba C et al (2019) Feasibility of individualised severe traumatic brain injury management using an automated assessment of optimal cerebral perfusion pressure: the COGiTATE phase II study protocol. BMJ Open 9(9):e030727
Bernard F, Gallagher C, Griesdale D, Kramer A, Sekhon M, Zeiler FA (2020) The Canadian High-Resolution Traumatic Brain Injury (CAHR-TBI) research collaborative. Can J Neurol Sci J Can Sci Neurol:1–20
Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, Shaffner DH (2008) Continuous measurement of autoregulation by spontaneous fluctuations in cerebral perfusion pressure: comparison of 3 methods. Stroke 39(9):2531–2537
Brady KM, Easley RB, Kibler K et al (2012) Positive end-expiratory pressure oscillation facilitates brain vascular reactivity monitoring. J Appl Physiol Bethesda Md 1985 113(9):1362–1368
Budohoski KP, Reinhard M, Aries MJH, Czosnyka Z, Smielewski P, Pickard JD, Kirkpatrick PJ, Czosnyka M (2012) Monitoring cerebral autoregulation after head injury. Which component of transcranial Doppler flow velocity is optimal? Neurocrit Care 17(2):211–218
Calviello L, Donnelly J, Cardim D, Robba C, Zeiler FA, Smielewski P, Czosnyka M (2018) Compensatory-reserve-weighted intracranial pressure and its association with outcome after traumatic brain injury. Neurocrit Care 28(2):212–220
Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD (1997) Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 41(1):11–17 discussion 17-19
Depreitere B, Güiza F, Van den Berghe G, Schuhmann MU, Maier G, Piper I, Meyfroidt G (2014) Pressure autoregulation monitoring and cerebral perfusion pressure target recommendation in patients with severe traumatic brain injury based on minute-by-minute monitoring data. J Neurosurg 120(6):1451–1457
Depreitere B, Güiza F, Van den Berghe G, Schuhmann MU, Maier G, Piper I, Meyfroidt G (2016) Can optimal cerebral perfusion pressure in patients with severe traumatic brain injury be calculated based on minute-by-minute data monitoring? Acta Neurochir Suppl 122:245–248
Donnelly J, Czosnyka M, Adams H et al (2019) Twenty-five years of intracranial pressure monitoring after severe traumatic brain injury: a retrospective, single-center analysis. Neurosurgery 85(1):E75–E82
Fraser CD, Brady KM, Rhee CJ, Easley RB, Kibler K, Smielewski P, Czosnyka M, Kaczka DW, Andropoulos DB, Rusin C (2013) The frequency response of cerebral autoregulation. J Appl Physiol Bethesda Md 1985 115(1):52–56
Govindan RB, Brady KM, Massaro AN, Perin J, Jennings JM, DuPlessis AJ, Koehler RC, Lee JK (2019) Comparison of frequency- and time-domain autoregulation and vasoreactivity indices in a piglet model of hypoxia-ischemia and hypothermia. Dev Neurosci:1–13
Güiza F, Depreitere B, Piper I et al (2015) Visualizing the pressure and time burden of intracranial hypertension in adult and paediatric traumatic brain injury. Intensive Care Med 41(6):1067–1076
Güiza F, Meyfroidt G, Piper I et al (2017) Cerebral perfusion pressure insults and associations with outcome in adult traumatic brain injury. J Neurotrauma 34(16):2425–2431
Higgins J, Green S (2008) Cochrane handbook for systematic review of interventions, 1st edn. Wiley-Blackwell
Howells T, Johnson U, McKelvey T, Enblad P (2015) An optimal frequency range for assessing the pressure reactivity index in patients with traumatic brain injury. J Clin Monit Comput 29(1):97–105
Jaeger M, Lang EW (2013) Cerebrovascular pressure reactivity and cerebral oxygen regulation after severe head injury. Neurocrit Care 19(1):69–73
Kim D-J, Czosnyka Z, Keong N, Radolovich DK, Smielewski P, Sutcliffe MPF, Pickard JD, Czosnyka M (2009) Index of cerebrospinal compensatory reserve in hydrocephalus. Neurosurgery 64(3):494–501 discussion 501-502
Lang EW, Czosnyka M, Mehdorn HM (2003) Tissue oxygen reactivity and cerebral autoregulation after severe traumatic brain injury. Crit Care Med 31(1):267–271
Lang EW, Kasprowicz M, Smielewski P, Santos E, Pickard J, Czosnyka M (2015) Short pressure reactivity index versus long pressure reactivity index in the management of traumatic brain injury. J Neurosurg 122(3):588–594
Lazaridis C, DeSantis SM, Smielewski P, Menon DK, Hutchinson P, Pickard JD, Czosnyka M (2014) Patient-specific thresholds of intracranial pressure in severe traumatic brain injury. J Neurosurg 120(4):893–900
Le Roux P, Menon DK, Citerio G et al (2014) The International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a list of recommendations and additional conclusions: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit Care 21(Suppl 2):S282–S296
Liu X, Donnelly J, Czosnyka M et al (2017) Cerebrovascular pressure reactivity monitoring using wavelet analysis in traumatic brain injury patients: a retrospective study. PLoS Med 14(7):e1002348
Maas AIR, Menon DK, Steyerberg EW, Citerio G, Lecky F, Manley GT, Hill S, Legrand V, Sorgner A, CENTER-TBI Participants and Investigators (2015) Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery 76(1):67–80
Mathieu F, Khellaf A, Ku JC, Donnelly J, Thelin EP, Zeiler FA (2019) Continuous near-infrared spectroscopy monitoring in adult traumatic brain injury: a systematic review. J Neurosurg Anesthesiol. https://doi.org/10.1097/ANA.0000000000000620
Mathieu F, Khellaf A, Thelin EP, Zeiler FA (2019) Continuous thermal diffusion-based cerebral blood flow monitoring in adult traumatic brain injury: a scoping systematic review. J Neurotrauma 36(11):1707–1723
Mathieu F, Zeiler FA, Whitehouse DP, Das T, Ercole A, Smielewski P, Hutchinson PJ, Czosnyka M, Newcombe VFJ, Menon DK (2019) Relationship between measures of cerebrovascular reactivity and intracranial lesion progression in acute TBI patients: an exploratory analysis. Neurocrit Care. https://doi.org/10.1007/s12028-019-00885-3
Mathieu F, Zeiler FA, Ercole A et al (2020) Relationship between measures of cerebrovascular reactivity and intracranial lesion progression in acute TBI patients: a CENTER-TBI study. J Neurotrauma. https://doi.org/10.1089/neu.2019.6814
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269 W64
Radolovich DK, Aries MJH, Castellani G, Corona A, Lavinio A, Smielewski P, Pickard JD, Czosnyka M (2011) Pulsatile intracranial pressure and cerebral autoregulation after traumatic brain injury. Neurocrit Care 15(3):379–386
Sánchez-Porras R, Santos E, Czosnyka M, Zheng Z, Unterberg AW, Sakowitz OW (2012) “Long” pressure reactivity index (L-PRx) as a measure of autoregulation correlates with outcome in traumatic brain injury patients. Acta Neurochir 154(9):1575–1581
Sorrentino E, Budohoski KP, Kasprowicz M, Smielewski P, Matta B, Pickard JD, Czosnyka M (2011) Critical thresholds for transcranial Doppler indices of cerebral autoregulation in traumatic brain injury. Neurocrit Care 14(2):188–193
Sorrentino E, Diedler J, Kasprowicz M et al (2012) Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit Care 16(2):258–266
Stauss HM (2007) Identification of blood pressure control mechanisms by power spectral analysis. Clin Exp Pharmacol Physiol 34(4):362–368
Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, Chatfield D, Menon DK, Pickard JD (2002) Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med 30(4):733–738
Svedung Wettervik T, Howells T, Enblad P, Lewén A (2019) Temporal neurophysiological dynamics in traumatic brain injury: role of pressure reactivity and optimal cerebral perfusion pressure for predicting outcome. J Neurotrauma. https://doi.org/10.1089/neu.2018.6157
Svedung Wettervik T, Howells T, Ronne-Engström E, Hillered L, Lewén A, Enblad P, Rostami E (2019) High arterial glucose is associated with poor pressure autoregulation, high cerebral lactate/pyruvate ratio and poor outcome following traumatic brain injury. Neurocrit Care 31(3):526–533
Thelin EP, Raj R, Bellander B-M et al (2019) Comparison of high versus low frequency cerebral physiology for cerebrovascular reactivity assessment in traumatic brain injury: a multi-center pilot study. J Clin Monit Comput. https://doi.org/10.1007/s10877-019-00392-y
Timofeev I, Czosnyka M, Nortje J, Smielewski P, Kirkpatrick P, Gupta A, Hutchinson P (2008) Effect of decompressive craniectomy on intracranial pressure and cerebrospinal compensation following traumatic brain injury. J Neurosurg 108(1):66–73
Timofeev I, Carpenter KLH, Nortje J et al (2011) Cerebral extracellular chemistry and outcome following traumatic brain injury: a microdialysis study of 223 patients. Brain J Neurol 134(Pt 2):484–494
Tricco AC, Lillie E, Zarin W et al (2018) PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 169(7):467–473
Zeiler FA, Donnelly J, Calviello L, Smielewski P, Menon DK, Czosnyka M (2017) Pressure autoregulation measurement techniques in adult traumatic brain injury, part II: a scoping review of continuous methods. J Neurotrauma 34(23):3224–3237
Zeiler FA, Donnelly J, Menon DK, Smielewski P, Zweifel C, Brady K, Czosnyka M (2017) Continuous autoregulatory indices derived from multi-modal monitoring: each one is not like the other. J Neurotrauma 34(22):3070–3080
Zeiler FA, Donnelly J, Calviello L, Lee JK, Smielewski P, Brady K, Kim D-J, Czosnyka M (2018) Validation of pressure reactivity and pulse amplitude indices against the lower limit of autoregulation, part I: experimental intracranial hypertension. J Neurotrauma 35(23):2803–2811
Zeiler FA, Donnelly J, Cardim D, Menon DK, Smielewski P, Czosnyka M (2018) ICP versus laser Doppler cerebrovascular reactivity indices to assess brain autoregulatory capacity. Neurocrit Care 28(2):194–202
Zeiler FA, Donnelly J, Menon DK, Smielewski P, Hutchinson PJA, Czosnyka M (2018) A description of a new continuous physiological index in traumatic brain injury using the correlation between pulse amplitude of intracranial pressure and cerebral perfusion pressure. J Neurotrauma. https://doi.org/10.1089/neu.2017.5241
Zeiler FA, Donnelly J, Nourallah B, Thelin EP, Calviello L, Smielewski P, Czosnyka M, Ercole A, Menon DK (2018) Intracranial and extracranial injury burden as drivers of impaired cerebrovascular reactivity in traumatic brain injury. J Neurotrauma 35(14):1569–1577
Zeiler FA, Donnelly J, Smieleweski P, Menon D, Hutchinson PJ, Czosnyka M (2018) Critical thresholds of ICP derived continuous cerebrovascular reactivity indices for outcome prediction in non-craniectomized TBI patients: PRx, PAx and RAC. J Neurotrauma 35(10):1107–1115
Zeiler FA, Kim D-J, Cabeleira M, Calviello L, Smielewski P, Czosnyka M (2018) Impaired cerebral compensatory reserve is associated with admission imaging characteristics of diffuse insult in traumatic brain injury. Acta Neurochir 160(12):2277–2287
Zeiler FA, Lee JK, Smielewski P, Czosnyka M, Brady K (2018) Validation of intracranial pressure-derived cerebrovascular reactivity indices against the lower limit of autoregulation, part II: experimental model of arterial hypotension. J Neurotrauma 35(23):2812–2819
Zeiler FA, Ercole A, Beqiri E, et al (2019) Cerebrovascular reactivity is not associated with therapeutic intensity in adult traumatic brain injury: a CENTER-TBI analysis. Acta Neurochir. Wien
Zeiler FA, Ercole A, Beqiri E, et al (2019) Association between cerebrovascular reactivity monitoring and mortality is preserved when adjusting for baseline admission characteristics in adult TBI: A CENTER-TBI Study. J Neurotrauma. https://doi.org/10.1089/neu.2019.6808
Zeiler FA, Ercole A, Cabeleira M, et al (2019) Patient-specific ICP epidemiologic thresholds in adult traumatic brain injury: a CENTER-TBI validation study. J Neurosurg Anesth. In Press
Zeiler FA, Ercole A, Cabeleira M, Carbonara M, Stocchetti N, Menon DK, Smielewski P, Czosnyka M, CENTER-TBI High Resolution (HR ICU) Sub-Study Participants and Investigators (2019) Comparison of performance of different optimal cerebral perfusion pressure parameters for outcome prediction in adult traumatic brain injury: a collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) study. J Neurotrauma 36(10):1505–1517
Zeiler FA, Ercole A, Cabeleira M, Zoerle T, Stocchetti N, Menon DK, Smielewski P, Czosnyka M, CENTER-TBI High Resolution Sub-Study Participants and Investigators (2019) Univariate comparison of performance of different cerebrovascular reactivity indices for outcome association in adult TBI: a CENTER-TBI study. Acta Neurochir 161(6):1217–1227
Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD (2002) Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 106(14):1814–1820
Funding
FAZ receives research support from the Manitoba Public Insurance (MPI) Neurscience/TBI Research Endowment, the United States National Institutes of Health (NIH) through the National Institute of Neurological Disorders and Stroke (NINDS), the Canadian Institutes of Health Research (CIHR), the Canadian Foundation for Innovation (CFI), the University of Manitoba VPRI Research Investment Fund (RIF), the University of Manitoba Rudy Falk Clinician-Scientist Professorship, and the Health Sciences Centre Foundation Winnipeg. EPT receives research support from Svenska Sällskapet för Medicinsk Forskning (SSMF), Hjärnfonden (Mattsons Stiftelse) and Region Stockholm Funding (ALF). RR receives research support from Finska Läkaresällskapet and Medicinska Understödsföreningen Liv & Hälsa. AG is support by the Clinician Investigator program at the University of Manitoba. LF is supported by a Department of Surgery GFT Surgeons Research Grant from clinical earnings of GFT surgeons at the University of Manitoba, Winnipeg, Manitoba, Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable, given this is a review.
Additional information
Comments
Traumatic brain injury (TBI) affects millions of people each year and the most severe cases are treated in highly specialized neuro-intensive care units using multimodal neuromonitoring. Various invasive/non-invasive measurements are integrated to aid real-time assessment of brain physiology and guide therapeutic interventions to prevent secondary brain injury. The pressure reactivity index (PRx) has been proposed as a metric to continuously monitor cerebrovascular reactivity, but other similar indices with different advantages have been put forward. In this scoping review, the authors summarize the scientific evidence of non-PRx (ICP based) indices of cerebrovascular reactivity in adult TBI. As concluded in the paper, the literature is limited and primarily consists of retrospective investigations from a few centers. Currently, PRx-based optimization of cerebral perfusion pressure is investigated in a clinical feasibility trial (COGiTATE), but much further research is needed (including investigations in animal models) before any of the other indices can be put into clinical testing.
Alexander Lilja-Cyron
Copenhagen, Denmark
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Brain trauma
Rights and permissions
About this article
Cite this article
Hasen, M., Gomez, A., Froese, L. et al. Alternative continuous intracranial pressure-derived cerebrovascular reactivity metrics in traumatic brain injury: a scoping overview. Acta Neurochir 162, 1647–1662 (2020). https://doi.org/10.1007/s00701-020-04378-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04378-7