Abstract
Background
Paroxysmal sympathetic hyperactivity (PSH) is a less-known complication of traumatic brain injury (TBI). This study was done to assess the clinical features and outcome of patients who develop PSH following severe TBI.
Methods
A prospective observational study was done on patients, admitted in the intensive care unit, for treatment of severe TBI. The clinical characteristics and outcome of patients, with and without PSH, was compared. At the time of discharge, patients were assessed with the Disability Rating Scale (DRS), and at 6 months with the Glasgow Outcome Score Extended (GOSE).
Results
The incidence of PSH was 8 % (29/343). Tachycardia, hypertension, and sweating were seen in all of the patients. Tachypnea was seen in 24 (82.8 %), hyperthermia in 28 (96.6 %), and posturing in 13 (44.8 %) patients. Thirteen (44.8 %) patients had all six symptoms of PSH. Follow-up data were available for 23 (79.3 %) patients. At the end of 6 months, 14 (60.9 %) patients had died, seven (30.4 %) were severely disabled, and two (8.7 %) were moderately disabled. There was a significant correlation of GOSE with the number of symptoms of PSH (Spearman’s rho = 0.502, p = 0.015). The patients with PSH had significantly higher DRS scores at discharge, 25.3 vs. 19.9, p < 0.001; higher mortality at 6 months 60.9 vs. 30.4 %, p < 0.001; and higher proportions with unfavorable outcome.
Conclusions
Presence of PSH in patients with severe TBI was associated with prolonged hospital stay, poorer DRS at discharge, more deaths, and unfavorable outcome. The number of symptoms of PSH had a significant effect on outcome at 6 months.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autonomic dysfunction, known by various names, is a less-known and less-studied complication of traumatic brain injury (TBI). The current consensus is to adopt the term “paroxysmal sympathetic hyperactivity (PSH)” to describe the syndrome of simultaneous, paroxysmal transient increases in sympathetic and motor activity recognized in a subgroup of survivors of severe acquired brain injury [3]. Though PSH can arise from any causes of acquired brain injury, the majority of published cases resulted from traumatic brain injury (TBI) (79.4 %), followed by hypoxic brain injury (9.7 %) and stroke (5.4 %) [13]. The reported incidence of PSH in TBI ranges from 8 to 33 % [12]. It has been described in both adults and children with severe TBI [6, 8].
PSH is characterized by episodes of autonomic hyper-responsiveness to non-noxious stimuli. The episodes comprise various combinations of hyperthermia, hypertension, tachycardia, tachypnea, increased muscle tone, sweating, and other symptoms of sympathetic hyperactivity [15, 16]. A caution of remark is required before labeling the diagnosis of PSH, as an isolated feature of PSH may be present in patients with TBI due to various causes like seizures, sepsis, hypoxia, hypoglycemia, and pain [16]. PSH often occurs in the immediate period, i.e., 7 days after an injury, with fewer cases continuing into rehabilitation. Also, patients with PSH remain in a low-response state for weeks to months after injury [14]. Whether the early recognition and management of PSH determines the outcome of patients with TBI is not certain.
We conducted this study to look for the incidence of PSH in our patients with TBI, its clinical spectrum, and its impact on outcome.
Methods
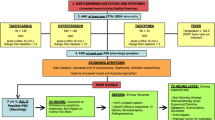
This prospective observational study included patients admitted to the neurosurgical intensive care unit between September 2013 and November 2015 for the treatment of severe TBI. An approval and clearance from the institution ethics committee and informed written consent from the relatives of the patients were obtained. Adult patients more than 18 years old with severe TBI, defined as post resuscitation Glasgow Coma Scale (GCS) 3 to 8 were screened for symptoms of PSH. The PSH was defined as episodes of simultaneous occurrence of four out of following six symptoms with at least one cycle per day for at least three consecutive days, and return to baseline in absence of episodes.
-
Temperature of >38.5 °C
-
Hypertension with systolic blood pressure >130 mmHg
-
Tachycardia with pulse rate of >100 beats per minute
-
Tachypnea with respiratory rate of >30 breaths per minute
-
Increased muscle tone, rigidity, dystonia, or decorticate/decerebrate posturing
-
Profuse sweating
The PSH assessment measure (PSH-AM) proposed by the consensus on conceptual definition, nomenclature, and diagnostic criteria for PSH was also applied to confirm the diagnosis of PSH [3]. The PSH-AM comprised “clinical feature scale” and “diagnosis likelihood tool”. The clinical features included were heart rate, respiratory rate, systolic blood pressure, temperature, sweating, and posturing during episode. Depending on severity, each feature was scored from 0 to 3. The features included in diagnosis likelihood tool in PSH-AM were:
-
Clinical features occurring simultaneously
-
Episodes being paroxysmal in nature
-
Sympathetic over-reactivity to normally non-painful stimuli
-
Features persisting ≥3 consecutive days
-
Features persisting ≥2 weeks post-brain injury
-
Features persisting despite treatment of alternative differential diagnoses
-
Medication administered to decrease sympathetic features
-
≥2 episodes daily
-
Absence of parasympathetic features during episodes
-
Absence of other presumed cause of features
-
Antecedent acquired brain injury
Each diagnosis likelihood tool was scored 1. Both clinical feature scale and diagnosis likelihood tool were added to give the total score. The diagnosis of PSH was based on combined total score as follows: unlikely <8, possible 8–16, and probable >17. Only patients with a PSH-AM score >17 were included in the study.
Patients with the following were excluded: injury to chest, abdomen, long bones, or spinal cord; on inotropes or other medications affecting autonomic nervous system, preexisting illness affecting nervous system, diabetes, cardiac arrhythmias, alcohol withdrawal, seizures, and sepsis. The exclusion of seizures was made by clinical observation. The exclusion of sepsis was made by negative bacterial cultures of blood, urine, and tracheal aspirate, as and when required. Episodes similar to PSH, during triggers like application of painful stimulus for Glasgow Coma Scale (GCS) assessment, tracheal suction, catheter insertion, weaning from ventilator, and extubation were not included.
The demographic, clinical, and computerized tomography (CT) scan details of the patients were recorded. Magnetic resonance imaging (MRI) of the brain was not done for patients. All patients were evaluated for development of symptoms suggestive of PSH. Two investigators (MJ and DS) confirmed the diagnosis of PSH, and enrolled the patients in the study. The patients diagnosed to have PSH were monitored continuously, and started on injectable or oral clonidine. Clonidine was administered at a dosage of 2 μg/kg body weight for a maximum dosage of 200 μg twice a day. It was continued until symptoms subsided. Simultaneously, other medications and treatments, as required for the management of a patient with severe TBI, were continued, including cerebral decongestants, antiepileptics, antibiotics, analgesics, and sedatives. Hydration was taken care of with adequate fluid replacement. Antipyretics and tepid sponging were used to control fever. Following parameters of PSH were recorded for each patient: number of episodes of PSH per day, total number of days of PSH, number of symptoms of PSH, and total number of episodes of PSH. At the time of discharge, patients were assessed with the Disability Rating Scale (DRS), and at 6-month follow-up with Glasgow Outcome Score Extended (GOSE) [17, 19]. The clinical characteristics and outcome of patients with PSH was compared with a control group of 79 patients with isolated TBI, but without PSH, studied during the same period, from the same ICU, for validation of outcome measure (unpublished). The control group was matched for established prognostic parameters of outcome of severe TBI.
Statistical analysis
The data was analyzed using the statistical software SPSS (version 21). Descriptive statistics were expressed as percentages for the categorical variables, while mean and standard deviation were calculated for continuous variables. The correlation coefficients were calculated using the Spearman’s rho. Comparison of data was done using Mann–Whitney U test for continuous variables, and Chi-square test or mid-p exact test for categorical variables. The p < 0.05 was considered as significant.
Results
A total of 343 adult patients with severe (TBI) were admitted during the study period, out of which 29 (8 %) patients satisfied the diagnostic criteria for PSH. The mean age of the patients was 33.7 ± 12.3 years (range, 18–70), and the majority (93.1 %) of them were males. The median (1st interquartile) admission GCS was 5 (three), and the median (1st interquartile) motor score was 3 (two). Twenty-six (89.6 %) patients underwent surgery for evacuation of traumatic mass lesions.
Severity of PSH
The number of symptoms manifested in individual patients varied. Tachycardia, hypertension, and sweating were seen in all the patients. Tachypnea was seen in 24 (82.8 %) patients and hyperthermia in 28 (96.6 %) patients. Only 13 (44.8 %) patients had posturing and dystonia during PSH episode. Thirteen (44.8 %) patients had all six symptoms of PSH simultaneously (Table 1). The mean number of days during which episodes of PSH were seen was 10.7 ± 8 per patient, and the mean of number of episodes were 25.0 ± 23.1 per patient, during hospital stay. The mean number of episodes per patient per day was 2.2 ± 0.9. Two patients had 100 episodes of PSH lasting 25 and 43 days, respectively. There was a significant difference in heart rate, systolic blood pressure, respiratory rate, and temperature, in each patient, between baseline and during episodes of PSH.
Outcome
The in-hospital mortality was five out of 29 (17.2 %). The mean DRS score at discharge was 25.3 ± 3.6, indicating extreme vegetative state. The mean duration of ICU stay was 11.9 ± 11.1 days, and hospital stay was 25.6 ± 19.6 days. Twenty-three (79.3 %) out of 29 patients were available at follow-up after 6 months of discharge. At the end of 6 months, 14 (60.9 %) patients died, seven (30.4 %) were severely disabled, and two (8.7 %) moderately disabled. None of the patients with PSH had good recovery. A comparison of patients with and without PSH was done. There was no difference in age, gender, admission GCS, CT scan classification of lesions, and need for surgical evacuation of traumatic mass lesion between these two groups. However, the patients with PSH had significantly lower motor scores (3 (2) vs. 4 (3), p = 0.03). There was no difference in in-hospital mortality and duration of ICU stay, however the duration of hospital stay was significantly longer in patients with PSH (25.5 ± 19.5 vs. 12.1 ± 11.1 days, p < 0.001). On comparing outcome, the patient with PSH had significantly higher DRS scores at discharge (25.3 ± 3.6 vs. 19.9 ± 4.7, p < 0.001); higher mortality at 6 months (60.9 vs. 30.4 %, p < 0.001); and higher proportions with unfavorable outcome (death, vegetative state or severe disability) (91.3 vs. 60.8 %, p < 0.001) (Table 2).
With regards to outcome of severity of PSH, a significant correlation of GOSE with the number of symptoms of PSH was seen (Spearman’s rho = 0.502, p = 0.015); however there was no significant correlation of outcome with mean number of episodes per day (Spearman’s rho = −0.13, p = 0.544), total number of episodes of PSH (Spearman’s rho = 0.005, p = 0.98), and number of days of PSH (Spearman’s rho = 0.07, p = 0.79) (Figs. 1 and 2)
Discussion
Though PSH is a well-known entity, only a few studies have been published reporting the incidence, clinical features, and outcome of PSH after severe TBI. In these studies, there has been a wide variation in the incidence of PSH ranging from 8-33 % [5, 12]. This could be explained by the lack of consensus in diagnosis, as isolated symptoms of PSH can be seen in various conditions like seizures, sepsis, hypoxia, hypoglycemia, and pain [16]. On employing a strict diagnostic criteria, we found that the incidence of PSH is not as high, as reported in some of the studies, indicating that we should avoid over-diagnosing PSH. The recently proposed PSH-AM is a useful tool for diagnosis, as it eliminates PSH-like symptoms due to alternate diagnosis [3].
With regards to the frequency of PSH episodes, the first report was by Fernandez-Ortega et al. [5, 8]. They observed that 18 (10.1 %) out of 179 patients with severe TBI experienced PSH, and the frequency of PSH episodes was 5.6 per day [8]. The frequency was lower in our study with the mean being 2.27 ± 0.88 per day per patient.
The duration of PSH varies between affected individuals, with PSH lasting up to 7 days post-injury, occurring in 25–33 % of survivors of severe TBI. Fernandez-Ortega et al. observed that all patients continued to experience episodes on discharge from the ICU, with 80 % of these resolved at the 12-month review [8]. Another study reported that 8–14 % of all severe TBI subjects develop a more prolonged form of PSH [4]. Though we did not determine the duration of each episode (other studies reporting a range from few minutes to 2 h [8, 11, 18]), the mean duration of PSH was 10.72 ± 8 days in our study. Two of our patients had 100 episodes of PSH lasting 25 and 43 days, respectively, and both patients were dead at 6 months.
Although the clinical implications of short-duration sympathetic hyperactivity are uncertain [2], most reports associate prolonged PSH following severe TBI with worse outcome [1, 4, 7]. The cause of increased mortality in such patients could be non-response to medications, leading to prolonged duration of PSH, which results in derangement of metabolic profile, malnourishment, and progressive deterioration of neurological condition. However, other studies have not identified an association between the duration of PSH and outcome [9, 10]. Even in our study, we could not find any correlation of duration of PSH with outcome.
However, among all the parameters of severity, we found the number of symptoms to be significantly associated with outcome; which has not been reported earlier. We observed that out of ten patients with all six symptoms, only one survived with moderate disability at 6-month follow-up. Probably, the difficulty in managing all the symptoms simultaneously could have resulted in higher mortality, indicating that the number of symptoms is the most important indicator of severity of PSH, rather than the duration.
The treatment of PSH is not standardized. A number of medications are used but all have class 3 evidence of efficacy in controlling symptoms of PSH [14, 16]. Whether the symptom control results in a more favorable outcome is not known. However, it is logical that sympathetic hyperactivity can harm the body, hence symptom control is the immediate goal particularly in an intensive care setting. We used clonidine in addition to supportive therapy. As we did not have a control group of patients with PSH who did not receive clonidine, we cannot comment on the benefits of clonidine. Early and aggressive treatment of PSH may be a subject of future research.
Conclusions
The incidence of PSH in our study was 8 %. The presence of PSH in patients with severe TBI was associated with prolonged duration of hospital stay, poorer DRS at discharge, more deaths, and unfavorable outcome. The number of symptoms of PSH had a significant effect on outcome at 6 months. There is a need for a multicenter study with adoption of PSH-AM, and to identify the impact of each of the clinical features on outcome of patients. There is also the need for a protocol for the management of PSH.
References
Baguley IJ, Nicholls JL, Felmingham KL, Crooks J, Gurka J, Wade LD (1999) Dysautonomia after traumatic brain injury: a forgotten syndrome? J Neurol Neurosurg Psychiatry 67:39–43
Baguley IJ, Nott MT, Slewa-Younan S, Heriseanu RE, Perkes IE (2009) Diagnosing dysautonomia after acute traumatic brain injury: evidence for over responsiveness to afferent stimuli. Arch Phys Med Rehabil 90:580–6
Baguley IJ, Perkes IE, Fernandez-Ortega J-F, Rabinstein AA, Dolce G, Hendricks HT (2014) Paroxysmal sympathetic hyperactivity after acquired brain injury: consensus on conceptual definition, nomenclature, and diagnostic criteria. J Neurotrauma 31:1515–1520
Baguley IJ, Slewa-Younan S, Heriseanu RE, Nott MT, Mudaliar Y, Nayyar V (2007) The incidence of dysautonomia and its relationship with autonomic arousal following traumatic brain injury. Brain Inj 21:1175–81
Boeve BF, Wijdicks EF, Benarroch EE, Schmidt KD (1998) Paroxysmal sympathetic storms (“diencephalic seizures”) after severe diffuse axonal head injury. Mayo Clin Proc 73:148–52
Deepika A, Mathew MJ, Kumar SA, Devi BI, Shukla D (2015) Paroxysmal sympathetic hyperactivity in pediatric traumatic brain injury: a case series of four patients. Auton Neurosci 193:149–51
Dolce G, Quintieri M, Leto E, Milano M, Pileggi A, Lagani V, Pignolo L (2008) Dysautonomia and clinical outcome in vegetative state. J Neurotrauma 25:1079–82
Fernandez-Ortega JF, Prieto-Palomino MA, Garcia-Caballero M, Galeas-Lopez JL, Quesada-Garcia G, Baguley IJ (2012) Paroxysmal sympathetic hyperactivity after traumatic brain injury: clinical and prognostic implications. J Neurotrauma 29:1364–70
Fernandez-Ortega JF, Prieto-Palomino MA, Muñoz-López A, Lebrón-Gallardo M, Arias-Verdú D, García-Caballero M, Quesada-García G (2004) Dysautonomic seizures in patients admitted to an intensive care unit following severe traumatic brain injury. Rev Neurol 39:715–8
Fernandez-Ortega JF, Prieto-Palomino MA, Muñoz-López A, Lebron-Gallardo M, Cabrera-Ortiz H, Quesada-García G (2006) Prognostic influence and computed tomography findings in dysautonomic crises after traumatic brain injury. J Trauma 61:1129–33
Goddeau RP, Silverman SB, Sims JR (2007) Dexmedetomidine for the treatment of paroxysmal autonomic instability with dystonia. Neurocrit Care 7:217–20
Hinson HE, Sheth KN (2012) Manifestations of the hyperadrenergic state after acute brain injury. Curr Opin Crit Care 18:139–45
Perkes IE, Menon DK, Nott MT, Baguley IJ (2011) Paroxysmal sympathetic hyperactivity after acquired brain injury: a review of diagnostic criteria. Brain Inj 25:925–32
Pozzi M, Conti V, Locatelli F, Galbiati S, Radice S, Citerio G, Clementi E, Strazzer S (2014) Paroxysmal sympathetic hyperactivity in pediatric rehabilitation: clinical factors and acute pharmacological management. J Head Trauma Rehabil 30:357–63
Rabinstein AA (2007) Paroxysmal sympathetic hyperactivity in the neurological intensive care unit. Neurol Res 29:680–2
Rabinstein AA, Benarroch EE (2008) Treatment of paroxysmal sympathetic hyperactivity. Curr Treat Options Neurol 10:151–157
Sander A (2002) The Extended Glasgow Outcome Scale. In: Center for Outcome Measure in Brain Injury. http://www.tbims.org/. Accessed 7 Mar 2016
Srinivasan S, Lim CCT, Thirugnanam U (2007) Paroxysmal autonomic instability with dystonia. Clin Auton Res 17:378–81
Wright J (2000) The Disability Rating Scale. In: Center for Outcome Measure in Brain Injury http://www.tbims.org/combi/drs. Accessed 24 Apr 2015
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Mathew, M.J., Deepika, A., Shukla, D. et al. Paroxysmal sympathetic hyperactivity in severe traumatic brain injury. Acta Neurochir 158, 2047–2052 (2016). https://doi.org/10.1007/s00701-016-2934-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-2934-x