Abstract
Background
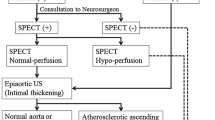
Although acetazolamide-challenged single-photon emission CT (SPECT) is recommended before carotid endarterectomy (CEA) and carotid artery stenting (CAS), given the relationship between preoperative decreased cerebrovascular reserve (CVR) and postoperative cerebral hyperperfusion syndrome (CHS), it is controversial whether all cases should be checked.
Methods
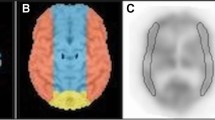
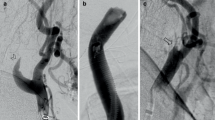
I-IMP-SPECT at rest was performed for 65 operative cases of carotid stenoses. At preoperative MR angiography we classified cases into two groups: G, featuring an anterior communicating artery with bilateral A1 with/without posterior communicating arteries; and P, a poor-escape-route group which did not match these criteria. Postoperative rCBF patterns were divided into two types: B, bilateral rCBF increase; and I, ipsilateral rCBF increase.
Results
Cases with high postoperative increase rate of rCBF were most frequently found in Group P and the Type I cases (p < 0.001). All four cases with hyperemia or hyperperfusion belonged to Group P. Only two out of 48 patients in Group G were Type I, both demonstrating a preoperative rCBF decrease rate more than 10 % as compared to the contralateral side.
Conclusions
From the present study, preliminary analysis of escape routes by preoperative MR angiography before surgical treatment of carotid stenosis is recommended and CVR investigation with acetazolamide-challenge SPECT should be considered for those relatively few cases with poor escape routes.



Similar content being viewed by others
References
Abou-Chebl A, Yadav JS, Reginelli JP, Bajzer C, Bhatt D, Krieger DW (2004) Intracranial hemorrhage and hyperperfusion syndrome following carotid arterystenting. J Am Coll Cardiol 43:1596–1601
Brott TG, Hobson RW II, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lal BK, Meschia JF, Investigators CREST (2010) Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 363:11–23
Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study (1995) JAMA 273: 1421–1428
Hosoda K, Fijita S, Kawaguchi T, Shose Y, Shibata Y, Tamaki N (1998) Influence of degree of carotid artery stenosis and collateral pathways and effect of carotid endarterectomy on cerebral vasoreactivity. Neurosurgery 42:988–994
Hosoda K, Kawaguchi T, Shibata Y, Kamei M, Kidoguchi K, Koyama J, Fujita S, Tamaki N (2001) Cerebral vasoreactivity and internal carotid artery flow help to identify patients at risk for hyperperfusion after carotid endarterectomy. Stroke 32:1567–1573
Hosoda K, Kawaguchi T, Ishii K, Minoshima S, Kohmura E (2005) Comparison of conventional region of interest and statistical mapping method in brain single-photon emission computed tomography for prediction of hyperperfusion after carotid endarterectomy. Neurosurgery 57:32–41
Iida H, Itoh H, Nakazawa M, Hatazawa J, Nishimura H, Onishi Y, Uemura K (1994) Quantitative mapping of regional cerebral blood flow using Iodine-123-IMMP and SPECT. J Nucl Med 35:2019–1030
Iwata T, Mori T, Tajiri H, Nakazaki M (2011) Predictors of hyperperfusion syndrome before and immediately after carotid artery stenting in single-photon emission computed tomography and transcranial color-coded real-time sonography studies. Neurosurgery 68:649–656
Kaku Y, Yoshimura S, Kokuzawa J (2004) Factors predictive of cerebral hyperperfusion after carotid angioplasty and stent placement. AJNR Am J Neuroradiol 25:1403–1408
Karapanayiotides T, Meuli R, Devuyst G, Piechowski-Jozwiak B, Dewarrat A, Ruchat P, Von Segesser L, Bogoussiavsky J (2005) Postcarotid endarterectomy hyperperfusion or reperfusion syndrome. Stroke 36:21–26
Kuroda H, Ogasawara K, Hirooka R, Kobayashi M, Fujiwara S, Chida K, Ishigaki D, Otawara Y, Ogawa A (2009) Prediction of cerebral hyperperfusion after carotid endarterectomy using middle cerebral artery signal intensity in preoperative single-slab 3-dimensional time-of-flight magnetic resonance angiography. Neurosurgery 64:1065–1072
Morrish W, Grahovac S, Douen A, Cheung G, Hu W, Farb R, Kalapos P, Wee R, Hudon M, Agbi C, Richard M (2000) Intracranial hemorrhage after stenting and angioplasty of extracranial carotid stenosis. AJNR Am J Neuroradiol 21:1911–1916
North American Symptomatic Carotid Endarterectomy Trial Collaborators (1991) Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 325:445–453
Ogasawara K, Yukawa H, Kobayashi M, Mikami C, Konno H, Terasaki K, Inoue T, Ogawa A (2003) Prediction and monitoring of cerebral hyperperfusion after carotid endarterectomy using single-photon emission computerized tomography scanning. J Neurosurg 99:504–510
Ogasawara K, Inoue T, Kobayashi M, Endo H, Fukuda T, Ogawa A (2004) Pretreatment with the free radical scavenger edaravone prevents cerebral hyperperfusion after carotid endarterectomy. Neurosurgery 55:1060–1067
Ogasawara K, Sakai N, Kuroiwa T, Hosoda K, Ihara K, Toyoda K, Sakai C, Nagata I, Ogawa A, Japanese Society for Treatment at Neck in Cerebrovascular Disease Study Group (2007) Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting: retrospective review of 4494 patients. J Neurosurg 107:1130–1136
Saito H, Ogasawara K, Suzuki T, Kuroda H, Kobayashi M, Yoshida K, Kubo Y, Ogawa A (2011) Adverse effects of intravenous acetazolamide administration for evaluation of cerebrovascular reactivity using brain perfusion single-photon emission computed tomography in patients with major cerebral artery steno-occlusive diseases. Neurol Med Chir 51:479–483
Schomer DF, Marks MP, Steinberg GK, Johnstone IM, Boothroyd DB, Ross MR, Pelc NJ, Enzmann DR (1994) The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med 330:1565–1570
Takeuchi R, Sengoku T, Matsumura K (2006) Usefulness of fully automated constant ROI analysis software for the brain: 3DSRT and FineSRT. Radiat Med 24:538–544
Torigai T, Mase M, Ohno T, Katano H, Nishikawa Y, Sakurai K, Sasaki S, Toyama J, Yamada K (2011) Usefulness of dual and fully automated measurements of cerebral blood flow during balloon occlusion test of the internal carotid artery. J Stroke Cerebrovasc Dis. doi:10.1016/j.jstrokecerebrovasdis.2011.07.015
van Mook WNKA, Rennenberg RJMW, Schurink GW, van Oostenbrugge RJ, Mess WH, Hofman PAM (2005) Cerebral hyperperfusion syndrome. Lancet Neurol 4:877–888
Yadav JS, Wholey MH, Kunts RE, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Whitlow P, Strickman NE, Jaff MR, Popma JJ, Snead DB, Cutlip DE, Firth BG, Ouriel K, Stenting and angioplasty with protection in patients at high risk for endarterectomy investigators (2004) Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 351:1493–1501
Acknowledgments
We thank Norimasa Takayanagi, Daiichi Radioisotope Laboratory for his technical support regarding 3DSRT and FineSRT, and Naoko Abe, Kojiro Hirose and all other staff for their invaluable assistance with SPECT.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
Here is an excellent communication with a novel idea and hypothesis from an experienced carotid intervention group in Nagoya. The goal was to identify preoperatively anatomical arterial characteristics, specifically predictably poor runoff (the P group), that could predict potential postoperative hyperemia and hyperpefusion syndromes.
As a preamble, I should say that it has not been our policy to perform SPECT scans in the routine workup of carotid surgery patents. Our rate of postoperative intracerebral hemorrhage is vanishing small (almost zero) for unclear reasons, so we have not developed a practice strategy to predict at-risk patients. Katano and colleagues, however, with this sentinel work, have convinced me that a clearer understanding of the risks and a rational evaluation strategy are worthwhile.
To summarize succinctly, patients with a good escape route (G) based on collateral anatomy, had low risk of hyperperfusion clinically or by SPECT. Three quarters of their patients were G type patients. Only two of these developed ipsilateral hyperpefusion post-treatemnt (Group I). By contrast, poor-runoff patients (P) were at high risk to revert to the I group and to have clinical symptoms as well.
So I am convinced that these authors can predict in their practice which patients are at risk for hyperemia based on anatomy (P) and thus can narrow down the focus of SPECT studies based on preoperative MRA data. The question is, what should we do with the information? To me, patients needing CEA or CAS for established indications will receive treatment whether they are “P” patients or not. Perhaps I would be more aggressive about ICU care and strict blood pressure control postoperatively based on “P” status, or SPECT data, but strict BP control and ICU observation are already my practice standard. This may account for the fact that despite my imperfect knowledge of this pathophysiology, we essentially never seen postoperative hyperperfusion syndrome or intracerebral hemorrhage, even though we sometimes have patients anticoagulated postoperatively, for prosthetic heart valves or bilateral symptomatic carotid disease.
I have learned from the Nagoya team here, and I better understand the physiology of collateral circulation as a result, and this in itself is worthwhile to the readership.
Christopher Miranda Loftus
Philadelphia, USA
Rights and permissions
About this article
Cite this article
Katano, H., Mase, M., Sakurai, K. et al. Revaluation of collateral pathways as escape routes from hyperemia/hyperperfusion following surgical treatment for carotid stenosis. Acta Neurochir 154, 2139–2149 (2012). https://doi.org/10.1007/s00701-012-1498-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-012-1498-7