Abstract
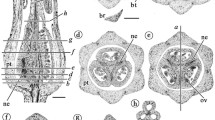
The Caryophyllales have the highest diversity in androecial patterns among flowering plants with stamen numbers ranging from 1 up to 4,000. Thanks to the recent progress in reconstructing the phylogeny of core Caryophyllales, questions of floral evolution, such as the origin and diversification of the androecium, can be readdressed. Caryophyllales are unique among core eudicots in sharing an androecial ring meristem or platform with centrifugal development of stamens and petals. Stamens are basically arranged in two whorls and evolution within the clade depends on the shift of either the antesepalous or the alternisepalous whorls to an upper position on the ring meristem and the reduction of the other. Four main developmental phenomena are responsible for the high diversity in androecial patterns: (1) the sterilisation of the outermost stamens through a division of common primordia; (2) the secondary addition of stamens by a centrifugal initiation of supernumerary stamens superimposed on a lower stamen number; (3) the pairwise displacement of alternisepalous stamens to the middle of the outer sepals and their potential fusion, or as part of a pluristaminate androecium; (4) the inversed sequence, reduction and loss of antesepalous stamens. Shifts in stamen numbers depend on pressures of the calyx and carpels and changes in the number of the latter. These patterns are expressed differently in the three main evolutionary lines of core Caryophyllales and are systematically relevant: (1) A basal grade of Caryophyllales, culminating with Caryophyllaceae, Amaranthaceae, Stegnosperma and Limeum, has the antesepalous stamens initiated in upper position on the ring meristem, and alternisepalous stamens are preferentially reduced. Among the antesepalous whorl there is a progressive loss of stamens following a sequence inversed to sepal initiation. Petaloid staminodes are formed by the radial division of outer stamens. (2) The raphide-clade and Molluginaceae are characterized by alternisepalous stamens in upper position on the ring meristem, with a trend to secondary stamen multiplication, and loss of antesepalous stamens. (3) The Portulacineae share the pattern of the raphide clade, but some taxa show shifts to an upper position on the ring meristem of either antesepalous or alternisepalous stamens, linked with secondary multiplications and reduction of either whorl. Different floral characters are plotted on a recent cladogram of Caryophyllales. The data show a consistent correlation between shifting carpel and stamen numbers independent of perianth evolution. Comparative data suggest that the basic androecium of Caryophyllales consists of two whorls of five stamens, linked with an absence of petals, and the evolution of the androecium is a combination of reductions and secondary multiplications of stamens with a highly predictive systematic value.











Similar content being viewed by others
References
Adamson RS (1958a) The South African species of Aizoaceae IV. Mollugo, Pharnaceum, Coelanthum and Hypertelis. J S Afr Bot 24:11–65
Adamson RS (1958b) The South African species of Aizoaceae V. Corbichonia. J S Afr Bot 24:67–69
Albert VA, Williams SE, Chase MW (1992) Carnivorous plants: phylogeny and structural evolution. Science 257:1491–1495
Applequist WL, Wallace RS (2001) Expanded circumscription of Didiereaceae and its subdivision into three subfamilies. Adansonia sér 3(25):13–16
Baillon H (1862) Observations sur les affinités du genre Barbeuia. Adansonia 3:312–317
Baillon H (1886) Organisation floral du Githago. Bull Mens Soc Linn Paris 1(76):603–604
Barthlott W, Hunt DR (1993) Cactaceae. In: Kubitzki K, Rohwer JG, Bittrich V (eds) The families and genera of vascular plants, vol 2. Springer, Berlin, pp 161–197
Batenburg LH, Moeliono BM (1982) Oligomery and vasculature in the androecium of Mollugo nudicaulis Lam. (Molluginaceae). Acta Bot Neerl 31:215–220
Batenburg LH, Geluk PCW, Moeliono BM (1984) Morphology and vascular system of the inflorescences of Mollugo nudicaulis Lam. and Hypertelis bowkeriana Sond. (Molluginaceae). Acta Bot Neerl 33:101–110
Bedell HG (1980) A taxonomic and morphological re-evaluation of Stegnospermaceae (Caryophyllales). Syst Bot 5:419–431
Behnke H-D (1976) Ultrastructure of sieve-element plastids in Caryophyllales (Centrospermae), evidence for the delimitation and classification of the order. Pl Syst Evol 126:31–54
Behnke H-D (1993) Further studies of the sieve-element plastids of the Caryophyllales including Barbeuia, Corrigiola, Lyallia, Microtea, Sarcobatus, and Telephium. Pl Syst Evol 186:231–243
Behnke H-D, Pop L, Sivarajan VV (1983a) Sieve-element plastids of Caryophylales: additional investigations with special reference to the Caryophyllaceae and Molluginaceae. Pl Syst Evol 142:109–115
Behnke H-D, Mabry TJ, Neumann P, Barthlott W (1983b) Ultrastructural, micromorphological and phytochemical evidence for a “central position” of Macarthuria (Molluginaceae) within the Caryophyllales. Pl Syst Evol 143:151–161
Bittrich V (1993a) Introduction to Centrospermae. In: Kubitzki K, Rohwer JG, Bittrich V (eds) The families and genera of vascular plants, vol 2. Springer, Berlin, pp 13–19
Bittrich V (1993b) Achatocarpaceae. In: Kubitzki K, Rohwer JG, Bittrich V (eds) The families and genera of vascular plants, vol 2. Springer, Berlin, pp 35–36
Bittrich V (1993c) Caryophyllaceae. In: Kubitzki K, Rohwer JG, Bittrich V (eds) The families and genera of vascular plants, vol 2. Springer, Berlin, pp 206–236
Bittrich V (1993d) Halophytaceae. In: Kubitzki K, Rohwer JG, Bittrich V (eds) The families and genera of vascular plants, vol 2. Springer, Berlin, pp 320–321
Bittrich V, Kühn U (1993) Nyctaginaceae. In: Kubitzki K, Rohwer JG, Bittrich V (eds) The families and genera of vascular plants, vol 2. Springer, Berlin, pp 473–486
Bogle AL (1970) The genera of Molluginaceae and Aizoaceae in the southeastern United States. J Arnold Arbor 51:431–462
Boke NH (1963) Anatomy and development of the flower and fruit of Pereskia pititache. Amer J Bot 50:843–858
Boke NH (1966) Ontogeny and structure of the flower and fruit of Pereskia aculeata. Amer J Bot 53:534–542
Brockington SF, Roolse A, Ramdial J, Moore MJ, Crawley S, Dhingra A, Hilu K, Soltis DE, Soltis PS (2009) Phylogeny of the Caryophyllales sensu lato: revisiting hypotheses on pollination biology and perianth differentiation in the core Caryophyllales. Int J Plant Sci 170:627–643
Brockington SF, Walker RH, Glover BJ, Soltis PS, Soltis DE (2011) Complex pigment evolution in the Caryophyllales. New Phytol 190:854–864
Brockington SF, Rudall PJ, Frohlich MW, Oppenheimer DG, Soltis PS, Soltis DE (2012) ‘Living stones’ reveal alternative petal identity programs within the core eudicots. Plant J 69:193–203
Brockington S, Dos Santos P, Glover B, Ronse De Craene LP (2013) Evolution of the androecium in Caryophyllales: insights from a paraphyletic Molluginaceae. Amer J Bot 100:1757–1778
Brown GK, Varadarajan GS (1985) Studies in Caryophyllales I: re-evaluation of classification of Phytolaccaceae s.l. Syst Bot 10:49–63
Buxbaum F (1961) Vorlaüfige Untersuchungen über Umfang, systematische Stellung und Gliederung der Caryophyllales (Centrospermae). Beitr Biol Pflanz 36:1–56
Carlquist S (2001) Wood and stem anatomy of Rhabdodendraceae is consistent with placement in Caryophyllales sensu lato. IAWA J 22:171–181
Carolin RC (1993) Portulacaceae. In: Kubitzki K, Rohwer JG, Bittrich V (eds) The families and genera of vascular plants, vol 2. Springer, Berlin, pp 544–555
Christin PL, Sage TL, Edwards EJ, Ogburn RM, Khoshravesh R, Sage RF (2011) Complex evolutionary transitions and the significance of C3–C4 intermediate forms of photosynthesis in Molluginaceae. Evolution 65:643–660
Clement J, Mabry T (1996) Pigment evolution in the Caryophyllales: a systematic overview. Botanica Acta 109:360–367
Cohn F (1913) Beiträge zur Kenntnis der Chenopodiaceen. Flora 106:51–89
Cuénoud P, Savolainen V, Chatrou LW, Powell M, Grayer RJ, Chase MW (2002) Molecular phylogenetics of Caryophyllales based on nuclear 18S rDNA and plastid rbcL, atpB, and matK DNA sequences. Amer J Bot 89:132–144
De Laet J, Clinckemaillie D, Jansen S, Smets E (1995) Floral ontogeny in the Plumbaginaceae. J Plant Res 108:289–304
Dickison WC, Miller RB (1993) Morphology and anatomy of the Malagasy genus Physena (Physenaceae), with a discussion of the relationships of the genus. Bull Mus Natl Hist Nat Paris IV, sect B, Adansonia 15:85–105
Donnison IS, Francis D (2002) Models of floral pattern in detached flowers of Silene coeli-rosa (L) Godr. (Caryophyllaceae). Bot J Linn Soc 140:229–235
Dos Santos P, Brockington S, Glover B, Ronse De Craene LP (2012) Micromorphological evidence for androecium origin of Claytonia (Montiaceae) petaloids. Modern Phytomorphology 1:23–25
Downie SR, Palmer JD (1994) A chloroplast DNA phylogeny of the Caryophyllales based on structural and inverted repeat restriction site variation. Syst Bot 19:236–252
Downie SR, Katz-Downie DS, Cho K-J (1997) Relationships in the Caryophyllales as suggested by phylogenetic analyses of partial chloroplast DNA ORF 2280 homolog sequences. Amer J Bot 84:253–273
Eckardt T (1967) Zur Blütenmorphologie von Dysphania plantaginella F. v.M. Phytomorphology 17:165–172
Eckardt T (1974) Vom Blütenbau der Centrospermen-Gattung Lophiocarpus Turcz. Phyton (Austria) 16:13–27
Eckardt T (1976) Classical morphological features of Centrospermous families. Pl Syst Evol 126:5–25
Eckert G (1966) Entwicklungsgeschichtliche und blütenanatomische Untersuchungen zum Problem der Obdiplostemonie. Bot Jahrb Syst 85:523–604
Eichler AW (1878) Blütendiagramme 2. Wilhelm Engelmann, Leipzig
Endress PK (2003) Morphology and angiosperm systematics in the molecular era. Bot Rev 68:545–570
Endress PK (2010) Flower structure and trends in evolution in eudicots and their major subclades. Ann Missouri Bot Gard 97:541–583
Endress M, Bittrich V (1993) Molluginaceae. In: Kubitzki K, Rohwer JG, Bittrich V (eds) The families and genera of vascular plants, vol 2. Springer, Berlin, pp 419–426
Endress PK, Matthews ML (2006) Elaborate petals and staminodes in eudicots: diversity, fuhction, and evolution. Org Div Evol 6:257–293
Endress PK, Matthews ML (2012) Progress and problems in the assessment of flower morphology in higher-level systematics. Pl Syst Evol 298:257–276
Erbar C, Leins P (2006) Floral ontogeny and systematic position of the Didiereaceae. Pl Syst Evol 261:165–185
Fay MF, Cameron KM, Prance GT, Lledó MD, Chase MW (1997) Familial relationships of Rhabdodendron (Rhabdodendraceae): plastid rbcL sequences indicate a caryophyllid placement. Kew Bull 52:923–932
Fiedler H (1910) Beiträge zur Kenntnis der Nyctaginaceen. Bot Jahrb Syst 44:572–605
Fior S, Karis PO, Casazza G, Minuto L, Sala F (2006) Molecular phylogeny of the Caryophyllaceae (Caryophyllales) inferred from chloroplast matK and nuclear rDNA ITS sequences. Amer J Bot 93:399–411
Franz E (1908) Beiträge zur Kenntnis der Portulacaceen und Basellaceen. Bot Jahrb Syst 42, Beibl 97:1–28
Friedrich H- C (1956) Studien über die natürliche Verwandtschaft der Plumbaginales und Centrospermae. Phyton (Austria) 6:220–263
Greenberg AK, Donoghue MJ (2011) Molecular systematics and character evolution in Caryophyllaceae. Taxon 60:1637–1652
Guaglianone R (1987) Phytolaccaceae. In: Burkart A (ed) Flora illustrada de entre Rios (Argentina) Tome VI, part III. Buenos Aires, Coleccion Cientifica del I.N.T.A., pp 209–225
Hakki MI (1972) Blütenmorphologische und embryologische Untersuchungen an Chenopodium capitatum und Chenopodium foliosum sowie weiteren Chenopodiaceae. Bot Jahrb Syst 92:178–330
Harbaugh DT, Nepokroeff M, Rabeler RK, McNeill J, Zimmer EA, Wagner WL (2010) A new lineage-based classification of the family Caryophyllaceae. Int J Plant Sci 171:185–198
Hardenack S, Ye D, Saedler H, Grant S (1994) Comparison of MADS box gene expression in developing male and female flowers of the dioecious plant White Campion. Plant Cell 6:1775–1787
Harris EM, Horn JW, Wagner WL (2012) Floral development of the divergent endemic Hawaiian genus Schiedea (Caryophyllaceae), with special emphasis on the floral nectaries. Taxon 61:576–591
Hartmann HEK (1993) Aizoaceae. In: Kubitzki K, Rohwer JG, Bittrich V (eds) The families and genera of vascular plants, vol 2. Springer, Berlin, pp 37–69
Haskell G (1949) Variation in the number of stamens in the common chickweed. J Genetics 49:291–301
Hassan NMS, Meve U, Liede-Schumann S (2005) Seed coat morphology of Aizoaceae-Sesuvioideae, Gisekiaceae and Molluginaceae and its systematic significance. Bot J Linn Soc 148:189–206
Hauman L (1951) Aizoaceae. Flore du Congo Belge et du Ruanda-Urundi. In: Spermatophytes, vol 2 Brussels: 100–117
Hershkowitz MA (1993) Revised circuscriptions and subgeneric taxonomies of Calandrinia and Montiopsis (Portulacaceae) with notes on phylogeny of the Portulacaceous alliance. Ann Missouri Bot Gard 80:333–365
Hofmann U (1973) Centrospermen-Studien 6: morphologische Untersuchungen zur Umgrenzung und Gliederung der Aizoaceen. Bot Jahrb Syst 93:247–324
Hofmann U (1977) Centrospermen-Studien 9: Die Stellung von Stegnosperma innerhalb der Centrospermen. Ber Deutsch Bot Ges 90:39–52
Hofmann U (1993). Gisekia: Blütenmorphologie und Entwicklungsgeschichte, systematische Folgerungen. In: Fürnkranz D, Schantl H (eds) Kurzfassungen 11 Symposium Morphologie, Anatomie und Systematik Salzburg 1993, p 17
Hofmann U (1994) Flower morphology and ontogeny. In: Behnke H-D, Mabry TD (eds) Caryophyllales. Evolution and Systematics, Springer Verlag, Berlin, pp 123–166
Ihlenfeldt HD (1960) Entwicklungsgeschichtliche, morphologische und systematische Untersuchungen an Mesembryanthemen. Feddes Repert 63:1–104
Joshi AC (1932) Dédoublement of stamens in Achyranthes aspera. Linn. J Indian Bot Soc 11:335–339
Joshi AC, Sita Rama Rao V (1934) Vascular anatomy of the flowers of four Nyctaginaceae. J Indian Bot Soc 13:169–186
Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donoghue MJ (2002) Plant systematics: a phylogenetic approach, 2nd edn. Sinauer, Sunderland 576 pp
Kadereit G, Borsch T, Weising K, Freitag H (2003) Phylogeny of Amaranthaceae and Chenopodiaceae and the evolution of C4 photosynthesis. Int J Plant Sci 164:959–986
Klak C, Khunou A, Reeves G, Henderson T (2003) A phylogenetic hypothesis for the Aizoaceae (Caryophyllales) based on four plastid DNA regions. Amer J Bot 90:1433–1445
Kühn U (1993) Chenopodiaceae. In: Kubitzki K, Rohwer JG, Bittrich V (eds) The families and genera of vascular plants, vol 2. Springer, Berlin, pp 253–281
Lacroix C, Sattler R (1988) Phyllotaxis theories and tepal-stamen superposition in Basella rubra. Amer J Bot 75:906–917
Lamb-Frye AS, Kron KA (2003) Phylogeny and character evolution in Polygonaceae. Syst Bot 21:17–29
Leins P, Erbar C (1994) Putative origin and relationships of the order from the viewpoint of developmental flower morphology. In: Behnke H-D, Mabry TD (eds) Caryophyllales evolution and systematics. Springer, Berlin, pp 303–316
Leins P, Erbar C (2003) Floral developmental features and molecular data in plant systematics. In: Stuessy TF, Mayer V, Hörandl E (eds) Deep morphology: towards a renaissance of morphology in plant systematics. ARG Gantner Verlag, Liechtenstein, pp 81–105
Leins P, Schwitalla S (1985) Studien an Cactaceen-Blüten I. Einige Bermerkungen zur Blütenentwicklung von Pereskia. Beitr Biol Pflanz 60:313–323
Leins P, Walter A, Erbar C (2001) Eine morphologische Interpretation der Caryophyllaceen-Kronblätter. Bot Jahrb Syst 123:355–367
Lepschi BJ (1996) A taxonomic revision of Macarthuria (Molluginaceae) in Western Australia. Nuytsia 11:37–54
Lüders H (1907) Systematische Untersuchungen über die Caryophyllaceen mit einfachem Diagramm. Bot Jahrb Syst 40 Beibl. 91:1–-37
Luo Y, Bian F-H, Luo Y-B (2012) Different patterns of floral ontogeny in dimorphic flowers of Pseudostellaria heterophylla (Caryophyllaceae). Int J Plant Sci 173:150–160
Lyndon RF (1978) Flower development in Silene: morphology and sequence of initiation of primordia. Ann Bot 42:1343–1348
Maddison DR, Maddison WP (2003) MacClade 4, Version 4.3. Sinauer, Sunderland, Mass
Meimberg H, Dittrich P, Bringmann G, Schlauer J, Heubl G (2000) Molecular phlogeny of Caryophyllidae s.l. based on MatK sequences with special emphasis on carnivorous taxa. Plant Biol 2:218–228
Milby TH (1980) Studies in the floral anatomy of Claytonia. Amer J Bot 67:1046–1050
Morton CM, Karol KG, Chase MW (1997) Taxonomic affinities of Physena (Physenaceae) and Asteropeia (Theaceae). Bot Rev 63:231–239
Müller K (1908) Beiträge zur Systematik der Aizoaceen. Bot Jahrb Syst 42, Beibl 97:54–72
Nyffeler R, Eggli U (2010) Disintegrating Portulacaceae: a new familial classification of the suborder Portulacineae (Caryophyllales) based on molecular and morphological data. Taxon 59:227–240
Olvera H, Flores Smets E, Vrijdaghs A (2008) Floral and inflorescence morphology and ontogeny in Beta vulgaris, with special emphasis on the ovary position. Ann Bot 102:643–651
Payer JB (1857) Traité d’organogénie comparée de la fleur: 748 p. 154 Pl. Victor Masson, Paris
Philipson WR (1993) Hectorellaceae. In: Kubitzki K, Rohwer J, Bittrich V (eds) Families and genera of vascular plants, vol 2. Springer, Berlin, pp 331–334
Pozner R, Cocucci A (2006) Floral structure, anther development, and pollen dispersal of Halophytum ameghinoi (Halophytaceae). Int J Plant Sci 167:1091–1098
Prance GT (2005) Rhabdodendraceae. In: Kubitzki K, Bayer C (eds) Families and genera of vascular plants vol 5. Springer, Berlin, pp 339–341
Puff C, Weber A (1976) Contributions to the morphology, anatomy and karyology of Rhabdodendron, and a reconsideration of the systematic position of the Rhabdodendraceae. Pl Syst Evol 125:195–222
Rohweder O (1965) Centrospermen-Studien 2: Entwicklung und morphologische Deutung des Gynöciums bei Phytolacca. Bot Jahrb Syst 84:509–526
Rohweder O (1967) Centrospermen-Studien 3: Blütenentwicklung und Blütenbau bei Silenoideen (Caryophyllaceae). Bot Jahrb Syst 86:130–185
Rohweder O (1970) Centrospermen-Studien 4: Morphology und Anatomie der Blüten, Früchte und Samen bei Alsinoideen und Paronychioideen s.lat. (Caryophyllaceae). Bot Jahrb Syst 90:201–271
Rohweder O, Huber K (1974) Centrospermen-Studien 7. Beobachtungen und Anmerkungen zur Morphologie und Entwicklungsgeschichte einiger Nyctaginaceen. Bot Jahrb Syst 94:327–359
Rohwer J (1993) Phytolaccaceae. In: Kubitzki K, Rohwer J, Bittrich V (eds) Families and genera of vascular plants, vol 2. Springer, Berlin, pp 506–515
Ronse De Craene LP (1990) Morphological studies in Tamaricales I: floral ontogeny and anatomy of Reaumuria vermiculata L. Beitr Biol Pflanz 65:181–203
Ronse De Craene LP (2004) Floral development of Berberidopsis corallina: a crucial link in the evolution of flowers in the core eudicots. Ann Bot 94:1–11
Ronse De Craene LP (2007) Are petals sterile stamens or bracts? The origin and evolution of petals in the core eudicots. Ann Bot 100:621–630
Ronse De Craene LP (2008) Homology and evolution of petals in the core eudicots. Syst Bot 33:301–325
Ronse De Craene LP (2010) Floral diagrams. An aid to understanding flower morphology and evolution. Cambridge University Press, Cambridge
Ronse De Craene LP, Brockington S (2013) Origin and evolution of petals in the angiosperms. Pl Ecol Evol 146:5–25
Ronse De Craene LP, Smets E (1991) The floral ontogeny of some members of the Phytolaccaceae (subfamily Rivinoideae) with a discussion of the evolution of the androecium in the Rivinoideae. Biol Jb Dodonaea 59:77–99
Ronse De Craene LP, Smets E (1992) Complex polyandry in the Magnoliatae: definition, distribution and systematic value. Nord J Bot 12:621–649
Ronse De Craene LP, Smets E (1994) Merosity: definition, origin and taxonomic significance. Pl Syst Evol 191:83–104
Ronse De Craene LP, Smets E (1995) The distribution and systematic relevance of the androecial character oligomery. Bot J Linn Soc 118:193–247
Ronse De Craene LP, Smets E (1998) Meristic changes in gynoecium morphology, exemplified by floral ontogeny and anatomy. In: Owens SJ, Rudall PJ (eds) Reproductive biology in systematics, conservation and economic botany. Royal Botanic Gardens, Kew, pp 85–112
Ronse De Craene LP, Smets EF (2001) Staminodes: their morphological and evolutionary significance. Bot Rev 67:351–402
Ronse De Craene LP, Stuppy W (2010) Floral development and anatomy of Aextoxicon punctatum (Aextoxicaceae-Berberidopsidales)—an enigmatic tree at the base of core eudicots. Int J Plant Sci 171:244–257
Ronse De Craene LP, Vanvinckenroye P, Smets EF (1997) A study of the floral morphological diversity in Phytolacca (Phytolaccaceae) based on early floral ontogeny. Int J Plant Sci 158:56–72
Ronse De Craene LP, Smets EF, Vanvinckenroye P (1998) Pseudodiplostemony, and its implications for the evolution of the androecium in the Caryophyllaceae. J Plant Res 111:25–43
Ronse De Craene LP, Volgin SA, Smets EF (1999) The floral development of Pleuropetalum darwinii, an anomalous member of the Amaranthaceae. Flora 194:189–199
Ross R (1982) Initiation of stamens, carpels and receptacle in the Cactaceae. Amer J Bot 69:369–379
Rudall PJ (2013) Identifying key features in the origin and early diversification of angiosperms. In: Ambrose BA, Purugganan MD (eds) The evolution of plant form. Ann Plant Rev 45:163–188
Rudall PJ, Bateman R (2010) Defining the limits of flowers: the challenge of distinguishing between the evolutionary products of simple versus compound strobili. Phil Trans R Soc B 365:397–409
Sanchez A, Schuster TM, Kron KA (2009) A large-scale phylogeny of Polygonaceae based on molecular data. Int J Plant Sci 170:1044–1055
Sattler R (1973) Organogenesis of flowers, a photographic text-atlas. University of Toronto Press, Toronto & Buffalo
Sattler R, Perlin L (1982) Floral development of Bougainvillea spectabilis Willd., Boerhaavia diffusa L. and Mirabilis jalapa L. (Nyctaginaceae). Bot J Linn Soc 84:161–182
Saunders ER (1937) Floral morphology, a new outlook, with special reference to the interpretation of the gynoecium I. Heffer & Sons, Cambridge
Schäferhoff B, Müller K, Borsch T (2009) Caryophyllales phylogenetics: disentangling Phytolaccaceae and Molluginaceae and description of Microteaceae as a new isolated family. Willdenowia 39:209–228
Schatz GE, Lowry II PP, Wolf A-E (1999) Endemic families of Madagascar IV. A synoptic revision of Asteropeia (Asteropeiaceae). Adansonia ser. 3, 21:255–268
Schölch M-F (1963) Die systematische Stellung der Didiereaceen im lichte neuer Untersuchungen über ihren Blütenbereich. Ber Deutsch Bot Ges 76: 49–55
Sharma HP (1954) Studies in the order Centrospermales I. Vascular anatomy of the flower of certain species of the Portulacaceae. J Indian Bot Soc 33:98–111
Sharma HP (1963) Studies in the order Centrospermales II. Vascular anatomy of the flower of certain species of the Molluginaceae. J Indian Bot Soc 42:19–32
Sherry RA, Eckard KJ, Lord EM (1993) Flower development in dioecious Spinacia oleracea (Chenopodiaceae). Amer J Bot 80:283–291
Smissen RD, Garnock-Jones PJ (2002) Relationships, classification and evolution of Scleranthus (Caryophyllaceae) as inferred from analysis of morphological characters. Bot J Linn Soc 140:15–29
Soltis DE, Smith SA, Cellinese N, Wurdack KJ, Tank DC, Brockington SF, Refulio-Rodriguez NF, Walker JB, Moore MJ, Carlsward BS, Bell CD, Latvis M, Crawley S, Black C, Diouf D, Xi Z, Rushworth CA, Gitzendanner mA, Sytsma KJ, Qiu Y-L, Hilu KW, Davis CC, Sanderson MJ, Beaman RS, Olmstead RG, Judd WS, Donoghue MJ, Soltis PS (2011) Angiosperm phylogeny: 17 genes, 640 taxa. Amer J Bot 98:704–730
Southwest Environmental Information Network (SEINet 2006) Stegnospermaceae. http://swbiodiversity.org/seinet/imagelib/imgdetails.php?imgid=254787
Stafford HA (1994) Anthocyanins and betalains: evolution of the mutually exclusive pathways. Plant Sci 101:91–98
Stannard BL (1988) Phytolaccaceae. In: Flora Zambesiaca, vol 9. http://apps.kew.org/efloras/namedetail.do?flora=fz&taxon=6698&nameid=17005
Sterk AA (1970) Reduction of the androecium in Spergularia marina (Caryophyllaceae). Acta Bot Neerl 19:488–494
Stevens PF (2001 onwards) Angiosperm Phylogeny Website. Version 12, July 2012 [and more or less continuously updated since]. http://www.mobot.org/MOBOT/research/APweb/
Thomson BF (1942) The floral morphology of the Caryophyllaceae. Amer J Bot 29:333–349
Townsend CC (1993) Amaranthaceae. In: Kubitzki K, Rohwer JG, Bittrich V (eds) The families and genera of vascular plants, vol 2. Springer, Berlin, pp 70–91
Vanvinckenroye P, Smets E (1996) Floral ontogeny of five species of Talinum and of related taxa (Portulacaceae). J Plant Res 109:387–402
Vanvinckenroye P, Smets E (1999) Floral ontogeny of Anacampseros subg. Anacampseros sect. Anacampseros (Portulacaceae). Syst Geogr Pl 68:173–194
Vanvinckenroye P, Cresens E, Ronse De Craene LP, Smets EF (1993) A comparative floral developmental study in Pisonia, Bougainvillea and Mirabilis (Nyctaginaceae) with special emphasis on the gynoecium and floral nectaries. Bull Jard Bot Nat Belg 62:69–96
Vanvinckenroye P, Ronse De Craene L, Smets E (1996) The floral development of Monococcus echinophorus (Phytolaccaceae). Can J Bot 75:1941–1950
Wagner WL, Harris EM (2000) A unique Hawaiian Schiedea (Caryophyllaceae: Alsinioideae) with only five fertile stamens. Amer J Bot 87:153–160
Walter H (1906) Die Diagramme der Phytolaccaceen. Bot Jahrb Syst 37, Beibl 85:1–57
Williams SE, Albert VA, Chase MW (1994) Relationships of Droseraceae: a cladistic analysis of rbcL sequence and morphological data. Amer J Bot 81:1027–1037
Acknowledgments
I thank Patricia Dos Santos and Sam Brockington for earlier discussions and for providing data used in this review. I am also grateful to Kester Bull and Sam Brockington for critical comments on an earlier version of the manuscript. Frieda Christie’s technical assistance with the SEM is acknowledged. I also thank the two reviewers for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Selected characters plotted on the phylogeny of Caryophyllales
-
1.
Perianth derivation (Fig. 4)
-
uniseriate, sepal derived: 0
-
biseriate, bract and sepal-derived: 1
-
biseriate, sepal and stamen-derived: 2
-
multiseriate, sepal and stamen-derived: 3
-
multiseriate, bract and sepal-derived: 4
-
-
2.
Calyx morphology
-
calyx sepaloid: 0
-
calyx completely petaloid: 1
-
calyx only petaloid at margins: 2
-
calyx only petaloid on adaxial side: 3
-
-
3.
Number of stamen whorls, including secondary increase (Fig. 5)
-
androecium of three whorls including outer staminodial petaloids: 0
-
androecium of two whorls including alternisepalous staminodial petaloids: 1
-
androecium of single whorl antesepalous: 2
-
androecium of single whorl alternisepalous (including the obhaplostemonous Plumbaginaceae): 3
-
Red dots indicate the presence of a secondary increase in stamen number superimposed on one, respectively two whorls.
-
-
4.
Position of upper stamens on androecial ring meristem (Fig. 6)
-
alternisepalous: 0
-
antesepalous: 1
-
No clear distinction between alternisepalous and antesepalous stamens arising at same level: 2
-
no obvious ring meristem: 3
-
-
5.
Initiation of the antesepalous stamen whorl (Fig. 7)
-
complete with acropetal initiation: 0
-
complete with inversed initiation: 1
-
incomplete with inversed initiation: 2
-
incomplete with acropetal initiation: 3
-
-
6.
Presence of staminodes
-
as petaloids: 0
-
as stublike structures: 1
-
as part of complex androecium: 2
-
staminodes absent: 3
-
-
7.
polyandry (also as red dots in Fig. 5)
-
division of complex primordia: 0
-
ring primordium: 1
-
absent: 2
-
-
8.
Carpel number (Fig. 8)
-
five-four: 0
-
three: 1
-
two: 2
-
one: 3
-
more than five: 4
-
-
9.
Carpel position
-
Isomerous—antesepalous: 0
-
Isomerous—alternisepalous: 1
-
Oligomerous—median or with two carpels abaxially: 2
-
Oligomerous—transversal or with two carpels adaxially: 3
-
Polymerous—pluricarpellate: 4
-
Appendix 2
See Table 2.
Rights and permissions
About this article
Cite this article
Ronse De Craene, L.P. Reevaluation of the perianth and androecium in Caryophyllales: implications for flower evolution. Plant Syst Evol 299, 1599–1636 (2013). https://doi.org/10.1007/s00606-013-0910-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-013-0910-y