Abstract
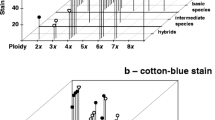
The chromosome analysis of Santolina rosmarinifolia subsp. rosmarinifolia, S. oblongifolia, S. semidentata subsp. semidentata, S. semidentata subsp. melidensis, S. canescens and the hybrid complex (S. rosmarinifolia subsp. rosmarinifolia, S. oblongifolia and their putative hybrids) shows that all the taxa are diploids (2n = 2x = 18; 18 + 1 or more B chromosomes, with 2n = 19, 20 only in the hybrid complex). The results show a conserved general structure of the karyotype (14m + 2sm + 2st), but in S. semidentata subsp. melidensis it is variable, with 14m + 2sm + 2st in ten individuals, 14m + (1m − 1sm) + (1 m − 1st) in nine individuals and 12m + (1m − 1sm) + (1m − 1st) + 2st + 1B in five individuals. Tetraploid individuals occurred in the diploid populations of S. rosmarinifolia subsp. rosmarinifolia and S. canescens, and their autopolyploid origin is discussed. Multivalent configurations at diakinesis, simple and double chromosome bridges and delayed disjunction of homologous and non-homologous chromosomes at anaphase I have negative effects on pollen stainability. The mean fructification percentage is moderate. The results suggest that the complex is a mosaic of introgressive hybrids.



Similar content being viewed by others
References
Abbott RJ (1992) Plant invasions, interspecific hybridization and the evolution of new plant taxa. Trends Ecol Evol 7:401–405
Allendorf FW, Leary RF, Spruell P, Wenburg JK (2001) The problems with hybrids: setting conservation guidelines. Trends Ecol Evol 16:613–622
Arnold ML (1997) Natural hybridization and evolution. Oxford series in ecology and evolution. Oxford University Press, Oxford
Baack EJ, Rieseberg LH (2007) A genomic view of introgression and hybrid speciation. Curr Opin Genet Dev 17:513–518
Barber JC, Finch CC, Francisco-Ortega J, Santos-Guerra A, Jansen RK (2007) Hybridization in Macaronesian Sideritis (Lamiaceae): evidence from incongruence of multiple independent nuclear and chloroplast sequence datasets. Taxon 56:74–88
Barton NH (2001) The role of hybridisation in evolution. Mol Ecol 10:551–568
Barton NH, Hewitt GM (1981) The genetic-basis of hybrid inviability in the grasshopper Podisma pedestris. Heredity 47:367–383
Barton NH, Hewitt GM (1989) Adaptation, speciation and hybrid zones. Nature 341:497–503
Barton NH, Hewitt GM (2001) The role of hybridisation in evolution. Mol Ecol 10:551–568
Bretagnolle F (2001) Pollen reduction and spontaneous polyploidization in diploid populations of Anthoxanthum alpinum. Biol J Linn Soc 72:241–247
Burton TL, Husband BC (2000) Fitness differences among diploids and tetraploids and their triploid progeny in Chamerion angustifolium (Onagraceae): mechanisms of inviability and implications for polyploidy evolution. Evolution 54:1182–1191
Cozzolino S, Nardellaa AM, Impagliazzoa S, Widmerb A, Lexerc C (2006) Hybridization and conservation of Mediterranean orchids: should we protect the orchid hybrids or the orchid hybrid zones? Biol Conserv 129:14–23
Darlington CD, Moffett AA (1930) Primary and secondary chromosome balance in Pyrus. J Genet 22:129–151
Dytham C (2003) Choosing and using statistics. A biologist’s guide, 2nd edn. Blackwell, Oxford
Ellstrand NC, Whitkus R, Rieseberg LH (1996) Distribution of spontaneous plant hybrids. Proc Natl Acad Sci USA 93:5090–5093
Felber F (1991) Establishment of the tetraploid cytotype in a diploid population: effect of relative fitness of the cytotypes. J Evol Biol 4:195–207
Ferreira VA (1985) Variabilidad y citogenética de los híbridos entre tres especies anuales de Helianthus. Mendeliana 7:13–30
Grafen A, Hails R (2003) Modern statistics for life science. Oxford University Press, Oxford
Grant PR, Grant BR (2002) Unpredictable evolution in a 30-year study of Darwin’s finches. Science 296:707–711
Guinea E (1970) Santolina europaeae. Anales Inst Bot A J Cavanilles 27:29–44
Gupta PP, Roy SK (1973) Primary and secondary chromosome association in Euryale ferox Salisb. Cytologia 38:645–649
Gustafsson M (1972) Distribution and effects of paracentric inversions in populations of Atriplex longipes. Hereditas 71:173–194
Harlan JR, deWet JM (1975) On Ö. Winge and prayer: the origins of polyploidy. Bot Rev 41:361–390
Harrison RG (1990) Hybrid zones: windows on evolutionary process. Oxford Surv Evol Biol 7:69–128
Husband BC (2004) The role of triploid hybrids in the evolutionary dynamics of mixed-ploidy populations. Biol J Linn Soc 82:537–546
Jackson RC (1965) A cytogenetic study of a three-paired race of Haplopappus gracilis. Am J Bot 52:946–953
Jackson RC, Casey J (1982) Cytogenetic analyses of autopolyploids: models and methods for triploids to octoploids. Am J Bot 69:487–501
Jacob KM (1957) Secondary association in the castor oil plant. Cytologia 22:380–392
Jones RN (1991) B-chromosomes drive. Am Nat 137:430–442
Jones RN, Houben A (2003) B chromosomes in plants: escapees from the A chromosome genome? Trends Plant Sci 8:417–423
Jordan A, Fourreau J (1869) Icones ad Floram Europae novo fundamento instaurandam spectantes. II. F. Savy, Paris
Jos JS, Vasudevan KN, Magno ML (1968) Structural hybridity in Typhonium cuspidatum Bl. Genét Ibér 20:1–11
Kempanna C, Riley R (1964) Secondary association between genetically equivalent bivalents. Heredity 19:289–296
Kim SC, Rieseberg LH (1999) Genetic architecture of species differences in annual sunflowers: implications for adaptive trait introgression. Genetics 153:965–977
Lacadena JR, Puertas MJ (1969) Secondary association of bivalents in allohexaploid, Aegilops triaristata Willd. 6x. Genét Ibér 21:191–209
Larson JL (1968) The species concept of Linnaeus. Isis 59:291–299
Levan A, Fredga K, Sandberg AA (1964) Nomenclature for centromeric position on chromosomes. Hereditas 52:201–220
Levin DA (1983) Polyploidy and novelty in flowering plants. Am Nat 122:1–25
Löve Á, Löve D (1975) Plant chromosomes. J. Cramer, Vaduz
Palestis BG, Burt A, Jones RN, Trivers R (2004) The distribution of B chromosomes across species. Cytogenet Gen Res 106:151–158
Raina SN, Khoshoo TK (1971) Cytogenetics of tropical bulbous ornamentals IV. Nature of triploidy in Crinum augustum. Cytologia 36:595–603
Rieseberg LH (1995) The role of hybridisation in evolution: old wine in new skins. Am J Bot 82:944–953
Rieseberg LH (1997) Hybrid origins of plant species. Annu Rev Ecol Syst 28:359–389
Rieseberg LH (2001) Chromosomal rearrangements and speciation. Trends Ecol Evol 16:351–358
Rieseberg LH, Ellstrand NC (1993) What can morphological and molecular markers tell us about plant hybridisation? Crit Rev Plant Sci 12:213–241
Rieseberg LH, Baird SJE, Gardner KA (2000) Hybridisation, introgression, and linkage evolution. Plant Mol Biol 42:205–224
Rieseberg LH, Kim S-C, Randell RA, Whitney KD, Gross BL, Lexer C, Clay K (2007) Hybridization and the colonization of novel habitats by annual sunflowers. Genetica 129:149–165
Riley R (1960) The secondary pairing of bivalents with genetically similar chromosomes. Nature 185:751–752
Rivero-Guerra AO (2008a) Cytogenetics, geographical distribution and pollen fertility of diploid and tetraploid cytotypes of Santolina pectinata Lag. (Asteraceae: Anthemideae). Bot J Linn Soc 156:657–667
Rivero-Guerra AO (2008b) Phenotypic differentiation of peripheral populations of Santolina rosmarinifolia L (Asteraceae: Anthemideae). Bot J Linn Soc 158:650–668
Rivero-Guerra AO (2008c) Cytogenetics, biogeography and biology of Santolina ageratifolia Barnades ex Asso (Asteraceae: Anthemideae). Bot J Linn Soc 157:797–807
Rivero-Guerra AO (2009a) Cytogenetics, geographical distribution, pollen stainability and fecundity of Santolina impressa Hoffmanns. & Link (Asteraceae: Anthemideae). Folia Geobot (in press)
Rivero-Guerra AO (2009b) Morphological variation within and between taxa of Santolina rosmarinifolia L. aggregate (Asteraceae: Anthemideae). Syst Bot (in press)
Rodríguez DJ (1996) A model for the establishment of polyploidy in plants. Am Nat 147:33–46
Rodriguez-Oubiña J, Ortiz S (1993) A new subspecies of Santolina rosmarinifolia L (Asteraceae) from serpentine soils in Central Galicia Iberian Peninsula). Bot J Linn Soc 111:457–462
Romero Zarco C (1986) A new method for estimating karyotype asymmetry. Taxon 35:526–530
Schweizer D, Ehrendorfer F (1983) Evolution of C-band patterns in Asteraceae-Anthemideae. Biol Zentr 102:637–655
Snow T (1963) Alcoholic hydrochloric acid-carmine as a stain for chromosomes in squash preparation. Stain Technol 38:9–13
Stebbins GL (1971) Chromosomal evolution in higher plants. Edward Arnold, London
Stein J, Quarin CL, Martínez EJ, Pessino SC, Ortiz JPA (2004) Tetraploid races of Paspalum notatum show polysomic inheritance and preferential chromosome pairing around the apospory-controlling locus. Theor Appl Genet 109:186–191
Stuessy TF, Weiss-Schneeweiss H, Keil DJ (2004) Diploid and polyploid cytotype distribution in Melampodium cinereum and M. leucanthum (Asteraceae, Heliantheae). Am J Bot 91:889–898
Sybenga J (1975) Meiotic configuration. Monographs on theoretical and applied genetics 1. Springer Verlag, Berlin
Thomas PT, Revell SH (1946) Secondary association and heterochromatic attraction. Ann Bot 10:159–164
Tjio JH, Levan A (1950) The use of oxyquinoline in chromosome analysis. Anales Est Exp Aula Dei 2:21–64
Valdés-Bermejo E, López G (1977) Aportaciones a la Flora Española. Anales Inst Bot Cavanilles 34:170–173
Valdés-Bermejo E, López G, Antúnez C (1981) Estudios cariológicos en especies españolas del género Santolina L. (Compositae). Anales Jard Bot Madrid 38:127–144
Wang JL, Tian L, Lee HS, Chen ML (2006) Non additive regulation of FRI and FLC loci mediates flowering-time variation in Arabidopsis allopolyploids. Genetics 173:965–974
Willkomm M (1859) Pugillus plantarum novarum peninsulae pyrenaicae. Linnaea 30:83–142
Willkomm M, Lange J (1870) Prodromus Florae Hispanicae. II. Typis e sumtibus librariae. E. Schweizerbart, Stuttgartiae
Acknowledgments
I thank the Instituto National de Meteorología for weather data on the study areas. I am grateful to Prof. P. Brandham, Prof. S. Talavera and Dr. A. Aparicio Martínez for comments and for their time, to Roger Churchill and P. Brandham for help with the English version of the manuscript, to Dr. F. J. Salgueiro for his collaboration on scanning the images, and to my friends J. Cabarga, J. M. Higueras Carranza, J. García López and A. Ojeda Ramírez for their help.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Rivero-Guerra, A.O. Cytogenetics, geographical distribution, pollen stainability and fecundity of five diploid taxa of Santolina rosmarinifolia L. aggregate (Asteraceae: Anthemideae). Plant Syst Evol 281, 17–34 (2009). https://doi.org/10.1007/s00606-009-0180-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-009-0180-x