Abstract
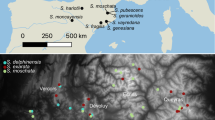
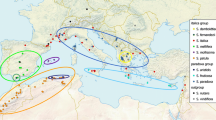
Eight variable regions (microsatellites, insertion/deletion and duplication regions) from the plastid DNA genome were analyzed for 91 populations belonging to Dactylorhiza majalis ssp. traunsteineri and closely related taxa. A total of 36 composite plastid haplotypes were found. The two dominating haplotypes had a clear geographic distribution suggesting at least two separate immigration routes into Scandinavia after the last glaciation: one southwestern route and one or two southeastern routes. D. majalis ssp. traunsteineri could not be clearly separated from any of the other taxa included in the study except for D. majalis ssp. sphagnicola. The morphologically similar taxa D. majalis ssp. traunsteineri, D. majalis ssp. lapponica and D. majalis ssp. russowii showed no genetic differentiation, and therefore we suggest an amalgamation of the three taxa into one broadly circumscribed subspecies; D. majalis ssp. lapponica. The plastid data also revealed incidents of hybridization and possible introgression between D. majalis ssp. lapponica and other members of the genus, e.g., D. incarnata.




Similar content being viewed by others
References
Aagaard SMD, Sastad SM, Greilhuber J, Moen AA (2005) Secondary hybrid zone between diploid Dactylorhiza incarnata ssp cruenta and allotetraploid D. lapponica (Orchidaceae). Heredity 94:488–496
Adams JM (1997) Global land environments since the last interglacial. Oak Ridge National Laboratory, TN. http://www.esd.ornl.gov/ern/qen/nerc.html
Adams JM, Faure H (eds) (1997) QEN members. Review and atlas of palaeovegetation: preliminary land ecosystem maps of the world since the last glacial maximum. Oak Ridge National Laboratory, TN. http://www.esd.ornl.gov/ern/qen/adams1.html
Andersson E (1994) On the identity of orchid populations: a morphometric study of the Dactylorhiza traunsteineri complex in eastern Sweden. Nord J Bot 14:269–275
Andersson E (1995) Age-related morphological differentiation among populations of Dactylorhiza traunsteineri (Orchidaceae) in eastern Sweden. Nord J Bot 15:127–137
Andersson E (1996) Morphological variation in the orchid Dactylorhiza traunsteineri. Scale and systematic implications. Doctoral dissertation, Uppsala University, Uppsala
Bandelt H-J, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Molec Biol Evol 16:37–48. http://www.fluxus-engineering.com
Baumann H, Künkele S (1988) Die Orchideen Europas. Kosmos Naturführer, Stuttgart
Baumann H, Künkele S, Lorenz R (2006) Orchideen Europas. Mit angrenzenden Gebieten. Ulmer Naturführer, Stuttgart
Berglund M (2004) Holocene shore displacement and chronology in Ångermanland, eastern Sweden, the Scandinavian glacio-isostatic uplift centre. Boreas 33:48–60
Berglund AN, Westerbergh A (2001) Two postglacial immigration lineages of the polyploid Cerastium alpinum (Caryophyllaceae). Hereditas 134:171–183
Bjurulf P (2005) Morphological variation in Dactylorhiza majalis ssp. sphagnicola and ssp. traunsteineri in different habitats. Svensk Bot Tidskr 99:124–132
Borgen L, Hultgård UM (2003) Parnassia palustris: a genetically diverse species in Scandinavia. Bot J Linn Soc 142:347–372
Brochmann C, Brysting AK, Alsos IG, Borgen L, Grundt HH, Scheen A-C, Elven R (2004) Polyploidy in arctic plants. Biol J Linn Soc 82:521–536
Cafasso D, Widmer A, Cozzolino S (2005) Chloroplast DNA inheritance in the orchid Anacamptis palustris using single-seed polymerase chain reaction. Heredity 96(1):66–70
Chase MW, Hills HG (1991) Silica gel: an ideal material for field preservation of leaf samples for DNA studies. Taxon 40:215–220
Corriveau JL, Coleman AW (1988) Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperm species. Amer J Bot 75(10):1443–1458
Cozzolino S, Cafasso D, Pellegrino G, Musacchio A, Widmer A (2003) Fine-scale phylogeographical analysis of Mediterranean Anacamptis palustris (Orchidaceae) populations based on chloroplast minisatellite and microsatellite variation. Molec Ecol 12:2783–2792
Delforge P (1995) Orchids of Britain and Europe. Harper Collins Publishers, London
Delforge P (2001) Guide des Orchidées d′Europe, d′Afrique du Nord et du Proche-Orient. Delachaux et Niestlé, Lausanne
Demesure B, Sodzi N, Petit RJ (1995) A set of universal primers for amplification of polymorphic non-coding regions of mitochondrial and chloroplast DNA in plants. Molec Ecol 4:129–131
de Soó R (1980) Dactylorhiza. In: Tutin TG et al. (eds) Flora Europea 5. Cambridge University Press, Cambridge, pp 333–337
Devos N, Tyteca D, Raspé O, Wesselingh RA, Jacquemart A-L (2003) Patterns of chloroplast diversity among western European Dactylorhiza species (Orchidaceae). Pl Syst Evol 243:85–97
Devos N, Raspé O, Oh S-H, Tyteca D, Jacquemart A-L (2006) The evolution of Dactylorhiza (Orchidaceae) allotetraploid complex: insights from nrDNA sequences and cpDNA PCR-RFLP data. Molec Phylogenet Evol 38:767–778
Doyle JJ, Doyle JH (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Dressler RL (1993) Phylogeny and classification of the orchid family. Cambridge University Press, Cambridge
Ekman S (1985) Dactylorhiza sphagnicola and D. traunsteineri with unspotted leaves found in E Uppland. Svensk Bot Tidskr 79:85–91
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online 1:47–50
Ferris C, King RA, Väinölä R, Hewitt GM (1998) Chloroplast DNA recognizes three refugial sources of European oaks and suggests independent eastern and western immigrations to Finland. Heredity 80:584–593
Grant V (1981) Plant speciation. Colombia University press, New York
Hansson S (1994) A study of Dactylorhiza lapponica. Svensk Bot Tidskr 88:17–28
Hedrén M (1996) Genetic differentiation, polyploidization and hybridization in northern European Dactylorhiza (Orchidaceae): evidence from allozyme markers. Pl Syst Evol 201:31–55
Hedrén M (2003) Plastid DNA variation in the Dactylorhiza incarnata/maculata polyploid complex and the origin of allotetraploid D. sphagnicola (Orchidaceae). Molec Ecol 12:2669–2680
Hedrén M, Fay MF, Chase MW (2001) Amplified fragment length polymorphisms (AFLP) reveal details of polyploid evolution in Dactylorhiza (Orchidaceae). Amer J Bot 88:1868–1880
Hedrén M, Nordström S, Hovmalm HAP, Pedersen HÆ, Hansson S (2007) Patterns of polyploid evolution in Greek marsh orchids (Dactylorhiza; Orchidaceae) as revealed by allozymes, AFLPs, and plastid DNA data. Amer J Bot 94:1205–1218
Heslop-Harrison J (1954) A synopsis of the dactylorchids of the British Isles. Berichte des Geobotanischen Forschungsinstituts Rübel 1953:53–82
Hewitt G (1996) Some genetic consequences of ice ages, and their role in divergence and speciation. Biol J Linn Soc 58:247–276
Hultén E (1950) Atlas of the distribution of vascular plants in NW Europe. Generalstabens litografiska anstalts förlag, Stockholm
Hylander N (1966) Nordisk kärlväxtflora II. Almquist och Wiksell, Stockholm
Jaarola M, Tegelström H (1995) Colonization history of north European field voles (Microtus agrestis) revealed by mitochondrial DNA. Molec Ecol 4:299–310
Jersáková J, Malinová T (2007) Spatial aspects of seed dispersal and seedling recruitment in orchids. New Phytol 176:448–459
Jordan WC, Courtney MW, Neigel JE (1996) Low levels of intraspecific genetic variation at a rapidly evolving chloroplast DNA locus in North American duckweeds (Lemnaceae). Amer J Bot 83:430–439
King RA, Ferris C (1998) Chloroplast DNA phylogeography of Alnus glutinosa (L) Gaertn. Molec Ecol 7:1151–1162
Krok ThOBN, Almquist S (1994) Svensk flora: Fanerogamer och ormbunksväxter. Liber, Stockholm
Kruskal JB (1964a) Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 29:1–27
Kruskal JB (1964b) Nonmetric multidimensional scaling: a numerical method. Psychometrika 29:28–42
Kullman L (1996) Norway spruce present in the Scandes mountains, Sweden at 8000 BP: new light on holocene tree spread. Glob Ecol Biogeogr lett 5:94–101
Lagercrantz U, Ryman N (1990) Genetic structure of Norway spruce (Picea abies): concordance of morphological and allozymic variation. Evolution 44:38–53
Löve A, Löve D (1974) Origin and the evolution of the arctic and alpine floras. In: Ives JD, Barry RG (eds) Arctic and alpine environments. Methuen, London, pp 571–603
Lowe A, Harris S, Ashton P (2004) Ecological genetics—design, analysis and application. Blackwell Publishing, Oxford
Lumaret R, Barrientos E (1990) Phylogenetic relationships and gene flow between sympatric diploid and tetraploid plants of Dactylis glomerata (Gramineae). Pl Syst Evol 169:81–96
Luttikhuizen PC, Stift M, Kuperus P, van Tienderen PH (2007) Genetic diversity in diploid vs. tetraploid Rorippa amphibia (Brassicaceae). Molec Ecol 16:3544–3553
Malm U, Prentice HC (2002) Immigration history and gene dispersal: allozyme variation in Nordic populations of the red campion, Silene dioica (Caryophyllaceae). Biol J Linn Soc 77:23–34
Malm U, Prentice HC (2005) Chloroplast DNA haplotypes in Nordic Silene dioica: postglacial immigration from the east and the south. Pl Syst Evol 250:27–38
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Mossberg B, Nilsson S (1987) Orkidéer- Europas vildväxande arter. Wahlström & Widstrand, Stockholm
Mossberg B, Stenberg L (2003) Den nya nordiska floran. Wahlström & Widstrand, Stockholm
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76:5269–5273
Nordal I, Jonsell B (1998) A phylogeographic analysis of Viola rupestris: three post-glacial immigration routes into the Nordic area? Bot J Linn Soc 128:105–122
Nordström S, Hedrén M (2007) Development of polymorphic nuclear microsatellite markers for polyploid and diploid members of the orchid genus Dactylorhiza. Molec Ecol Notes 7:644–647
Otto SP, Whitton J (2000) Polyploid incidence and evolution. Ann Rev Genet 34:401–437
Palmé AE, Su Q, Rautenberg A, Manni F, Lascoux M (2003) Postglacial recolonization and cpDNA variation of silver birch, Betula pendula. Molec Ecol 12:201–212
Pedersen HÆ, Hedrén M, Bateman RM (2003) Proposal to conserve the name Orchis majalis against O. elata, O. vestita and O. sesquipedalis (Dactylorhiza: Orchidinae: Orchidaceae). Taxon 52:633–634
Pillon Y, Fay MF, Hedrén M, Bateman RM, Devey DS, Shipunov AB, van der Bank M, Chase MW (2007) Evolution and temporal diversification of western European polyploid species complexes in Dactylohiza (Orchidaceae). Taxon 56:1185–1208
Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN (2001) Genera orchidacearum, 2: 1. Oxford University Press, Oxford
Rendell S, Ennos RA (2002) Chloroplast DNA diversity in Calluna vulgaris (heather) populations in Europe. Molec Ecol 11:69–78
Rohlf FJ (2005) NTSYSpc: numerical taxonomy system, ver. 2.20. Exeter Publishing, Ltd., Setauket
van Rossum F, Prentice HC (2004) Structure of allozyme variation in Nordic Silene nutans (Caryophyllaceae): population size, geographical position and immigration history. Biol J Linn Soc 81:357–371
Senghas K (1968) Taxonomische Übersicht der gattung Dactylorhiza Necker ex Nevski. In: Senghas K, Sundermann H (eds) Probleme der Orchideengattung Dactylorhiza. Jahresber Naturwiss Vereins Wuppertal 21–22:32–67
Shipunov AB, Fay MF, Chase MW (2005) Evolution of Dactylorhiza baltica (Orchidaceae) in European Russia: evidence from molecular markers and morphology. Bot J Linn Soc 147:257–274
Skrede I, Eidesen PB, Portela RP, Brochmann C (2006) Refugia, differentiation and postglacial migration in arctic-alpine Eurasia, exemplified by the mountain avens (Dryas octopetala L.). Molec Ecol 15:1827–1840
Soliva M, Widmer A (1999) Genetic and floral divergence among sympatric populations of Gymnadenia conopsea s.l. (Orchidaceae) with different flowering phenology. Int J Pl Sci 160:897–905
Soltis DE, Rieseberg LH (1986) Autopolyploidy in Tolmiea-menziesii (Saxifragaceae): Genetic insights from enzyme electrophoresis. Amer J Bot 73:310–318
Ståhlberg D (2007) Systematics, phylogeography and polyploid evolution in the Dactylorhiza maculata complex (Orchidaceae). Doctoral dissertation, Lund University, Lund
Stewart JR, Lister AM (2001) Cryptic northern refugia and the origins of the modern biota. Trends Ecol Evol 16:608–613
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Pl Molec Biol 17:1105–1109
Taberlet P, Swenson JE, Sandegren F, Bjärvall A (1995) Localization of a contact zone between two higly divergent mitochondrial DNA lineages of the brown bear (Ursus arctos) in Scandinavia. Conserv Biol 9:1255–1261
Tyler T, Prentice HC, Widén B (2002) Geographic variation and dispersal history in Fennoscandian populations of two forest herbs. Pl Syst Evol 233:47–64
Wolfe AD, Randle CP (2004) Recombination, heteroplasmy, haplotype polymorphism, and paralogy in plastid genes: implications for plant molecular systematics. Syst Bot 29:1011–1020
Acknowledgments
We would like to thank Sunniva Aagaard, Åse Bøilestad Breivik, Stefan Ericsson, Sven Hansson, Ursula Malm, Lennart Nordström, Tarmo Pikner, Mikko Piirainen, Mari Reitalu, David Ståhlberg, Taavo Tuulik, Kai Vahtra and Finn Wischmann for field assistance and/or collecting material. We also thank two anonymous reviewers for valuable comments. The study was supported by grants from Lunds Botaniska Förening to SN and from the Crafoord Foundation and the Swedish research council for environment, agricultural sciences and spatial planning, FORMAS (grant 2002-102) to MH.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Table 3
Rights and permissions
About this article
Cite this article
Nordström, S., Hedrén, M. Genetic differentiation and postglacial migration of the Dactylorhiza majalis ssp. traunsteineri/lapponica complex into Fennoscandia. Plant Syst Evol 276, 73–87 (2008). https://doi.org/10.1007/s00606-008-0084-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-008-0084-1