Abstract
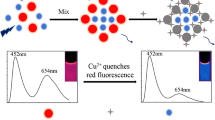
A new fluorescence sensing strategy has been developed. Four bimetallic nanoclusters, gold/silver, gold/copper, gold/molybdenum and gold/cobalt, were prepared using bovine serum albumin (BSA) as a reducing and stabilizing agent. The fluorescence properties of four nanoclusters were explored by solid-state UV and XPS. The gold/silver nanoclusters (BSA-Au/Ag NCs) with the best ratiometric fluorescence properties for gallic acid (GA) in plants were selected to realize the sensitive detection of GA. GA affected the conformation of BSA, thereby disrupting the luminescent environment of the nanoclusters, resulting in a pronounced fluorescence quenching at 566 nm. The ratiometric fluorescence signal (I566/I453) was used for trace detection of GA in plants. It has a wide response range of 1.25–40.0 μM and a low detection limit of 45.27 nM. GA was detected at 19.49 μM in the plant extract, and the spiked recoveries ranged from 96.09 to 104.6%. In addition, due to the non-toxic and biocompatible properties of BSA, BSA-Au/Ag NCs have also been validated for fluorescence imaging of plant tissues. It realized the comparison of GA content in different parts of plants and the difference of GA content in plants after abiotic stress. Therefore, the developed strategy offers potential application for the analytical study of active substances in plants.
Graphical Abstract









Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this article [and its supplementary information files].
References
Movahedi A, Almasi Zadeh Yaghuti A, Wei H, Rutland P, Sun W, Mousavi M, Li D, Qiang Z (2021) Plant secondary metabolites with an overview of populus. Int J Mol Sci 22:6890. https://doi.org/10.3390/ijms22136890
Shojaee MS, Moeenfard M, Farhoosh R (2022) Kinetics and stoichiometry of gallic acid and methyl gallate in scavenging DPPH radical as affected by the reaction solvent. Sci Rep 12:8765. https://doi.org/10.1038/s41598-022-12803-3
Rangani J, Panda A, Parida AK (2020) Metabolomic study reveals key metabolic adjustments in the xerohalophyte Salvadora persica L. during adaptation to water deficit and subsequent recovery conditions. Plant Physiol Biochem 150:180–195. https://doi.org/10.1016/j.plaphy.2020.02.036
Sukhikh S, Asyakina L, Korobenkov M, Skrypnik L, Pungin A, Ivanova S, Larichev T, Larina V, Krol O, Ulrikh E et al (2021) Chemical composition and content of biologically active substances found in Cotinus coggygria, Dactylorhiza maculata, Platanthera chlorantha growing in various territories. Plants-Basel 10:2806. https://doi.org/10.3390/plants10122806
Wang X, Wang J, Yang N (2007) Flow injection chemiluminescent detection of gallic acid in olive fruits. Food Chem 105:340–345. https://doi.org/10.1016/j.foodchem.2006.11.061
Pathak K, Das RJ, Gogoi N, Saikia R, Sarma H, Das A (2022) A validated high-performance thin-layer chromatography method for the simultaneous determination of quercetin and gallic acid in Annona reticulata L. Jpc-J Planar Chromat 35:35–41. https://doi.org/10.1007/s00764-022-00151-x
Chen L, Yang J, Chen W, Sun S, Tang H, Li Y (2020) Perovskite mesoporous LaFeO3 with peroxidase-like activity for colorimetric detection of gallic acid. Sens Actuators B 321:128642. https://doi.org/10.1016/j.snb.2020.128642
Sivakumar M, Pandi K, Chen S-M, Yadav S, Chen T-W, Veeramani V (2019) Highly sensitive detection of gallic acid in food samples by using robust NiAl2O4 nanocomposite materials. J. Electrochem. Soc. 166: B29-B34. https://doi.org/10.1149/2.0121902jes
Zhang Y, Ning L, Gao D, Jia D, Gu W, Liu X (2021) A highly sensitive upconversion nanoparticles@zeolitic imidazolate frameworks fluorescent nanoprobe for gallic acid analysis. Talanta 233:122588. https://doi.org/10.1016/j.talanta.2021.122588
Zhan D, Bian Z, Li H, Wang R, Fang G, Yao Q, Wu Z (2022) Novel detection method for gallic acid: a water soluble boronic acid-based fluorescent sensor with double recognition sites. Bioorg Med Chem Lett 57:128483. https://doi.org/10.1016/j.bmcl.2021.128483
Mir IA, Bhat MA, Muhammad Z, Rehman SU, Hafeez M, Khan Q, Zhu L (2019) Differential and comparative sensing modes of AIS and AIS@ZnS core-shell quantum dots towards bioanalytes. J Alloys Compd 811:151688. https://doi.org/10.1016/j.jallcom.2019.151688
Pan L, Li X, Zhang Q, Xu S, Yang L, Yang F, Jiang C (2022) A boric acid functional multi-emission metal organic frameworks-based fluorescence sensing platform for visualization of gallic acid. Chem Eng J 450:138283. https://doi.org/10.1016/j.cej.2022.138283
Liu Z, Wang M, Wu M, Li X, Liu H, Niu N, Li S, Chen L (2023) Volatile organic compounds (VOCs) from plants: from release to detection. Trends Anal Chem 158:116872. https://doi.org/10.1016/j.trac.2022.116872
Chinnabathini VC, Dingenen F, Borah R, Abbas I, van der Tol J, Zarkua Z, D'Acapito F, Nguyen THT, Lievens P, Grandjean D et al (2023) Gas phase deposition of well-defined bimetallic gold-silver clusters for photocatalytic applications. Nanoscale 15:6696–6708. https://doi.org/10.1039/d2nr07287d
Zhai Q, Xing H, Fan D, Zhang X, Li J, Wang E (2018) Gold-silver bimetallic nanoclusters with enhanced fluorescence for highly selective and sensitive detection of glutathione. Sens Actuators B 273:1827–1832. https://doi.org/10.1016/j.snb.2018.05.145
Zhai Q, Xing H, Zhang X, Li J, Wang E (2017) Enhanced electrochemiluminescence behavior of gold-silver bimetallic nanoclusters and its sensing application for mercury(II). Anal Chem 89:7788–7794. https://doi.org/10.1021/acs.analchem.7b01897
Singh C, Mehata AK, Tiwari P, Setia A, Malik AK, Singh SK, Tilak R, Muthu MS (2023) Design of novel bioadhesive chitosan film loaded with bimetallic gold-silver nanoparticles for antibiofilm and wound healing activity. Biomed Mater 18:025014. https://doi.org/10.1088/1748-605X/acb89b
Suo Z, Hou X, Chen J, Liu X, Liu Y, Xing F, Chen Y, Feng L (2020) Highly chiroptical detection with gold-silver bimetallic nanoclusters circularly polarized luminescence based on G-quartet nanofiber self-assembly. J Phys Chem C 124:21094–21102. https://doi.org/10.1021/acs.jpcc.0c06388
Mao J, Li J, Pei J, Liu Y, Wang D, Li Y (2019) Structure regulation of noble-metal-based nanomaterials at an atomic level. Nano Today 26:164–175. https://doi.org/10.1016/j.nantod.2019.03.008
Li D, Liu Q, Qi Q, Shi H, Hsu E-C, Chen W, Yuan W, Wu Y, Lin S, Zeng Y et al (2020) Gold Nanoclusters for NIR-II fluorescence imaging of bones. Small 16:2003851. https://doi.org/10.1002/smll.202003851
Huang T-H, Zhao F-Z, Hu Q-L, Liu Q, Wu T-C, Zheng D, Kang T, Gui L-C, Chen J (2020) Bisphosphine-stabilized gold nanoclusters with the crown/birdcage-shaped Au-11 cores: structures and optical properties. Inorg Chem 59:16027–16034. https://doi.org/10.1021/acs.inorgchem.0c02582
Sun H, Qing T, He X, Shangguan J, Jia R, Bu H, Huang J, Wang K (2019) Rapid synthesis of Au/Ag bimetallic nanoclusters with highly biochemical stability and its applications for temperature and ratiometric pH sensing. Anal Chim Acta 1070:88–96. https://doi.org/10.1016/j.aca.2019.04.029
Yang X, Yang Z, Tang F, Xu J, Zhang M, Choi MMF (2019) Structural and optical properties of penicillamine-protected gold nanocluster fractions separated by sequential size-selective fractionation. Beilstein J Nanotechnol 10:955–966. https://doi.org/10.3762/bjnano.10.96
Xu J, Zhang W, Lv P, Li F, Zhan X, Zhang Y, Liu X (2023) Improved fluorescence and photoelectrical properties of CsPbBr3 by Constructing heterojunctions under pressure. Small 19:202305870. https://doi.org/10.1002/smll.202305870
Attia Y, Samer M (2017) Metal clusters: New era of hydrogen production. Renew Sustain Energy Rev 79:878–892. https://doi.org/10.1016/j.rser.2017.05.113
Liu G, Zhou H, Che Q, Liu B, Li J, Cao B, Liu Z (2021) A novel phosphor of Cu+-doped PbBrOH: preparation, luminescence mechanism, and outstanding properties. J Mater Chem C 9:9178–9187. https://doi.org/10.1039/d1tc01855h
Chen L, Gharib M, Zeng Y, Roy S, Nandi CK, Chakraborty I (2023) Advances in bovine serum albumin-protected gold nanoclusters: from understanding the formation mechanisms to biological applications. Mater Today Chem 29:101460. https://doi.org/10.1016/j.mtchem.2023.101460
Ma X, Wen X, Toh Y-R, Huang K-Y, Tang J, Yu P (2014) Dynamic study on the transformation process of gold nanoclusters. Nanotechnology 25:445705. https://doi.org/10.1088/0957-4484/25/44/445705
Alom SE, Swaminathan R (2023) Protein charge transfer spectra in a monomeric protein with no lysine. Phys Chem Chem Phys 25:16626–16642. https://doi.org/10.1039/d2cp05836g
Vijayakumar S, Rowlette J, Schwaighofer A, Lendl B (2023) Laser-based mid-infrared spectroscopy for monitoring temperature-induced denaturation of bovine serum albumin and de-/stabilization effects of sugars. Anal Chem 95:6441–6447. https://doi.org/10.1021/acs.analchem.3c00489
Tian Z, Tian L, Shi M, Zhao S, Guo S, Luo W, Wang C, Tian Z (2020) Investigation of the interaction of a polyamine-modified flavonoid with bovine serum albumin (BSA) by spectroscopic methods and molecular simulation. J Photochem Photobiol B 209:111917. https://doi.org/10.1016/j.jphotobiol.2020.111917
Sannigrahi A, Chowdhury S, Nandi I, Sanyal D, Chall S, Chattopadhyay K (2019) Development of a near infrared Au-Ag bimetallic nanocluster for ultrasensitive detection of toxic Pb2+ ions in vitro and inside cells. Nanoscale Adv 1:3660–3669. https://doi.org/10.1039/c9na00459a
Liu Z, Wang X, Ren X, Li W, Sun J, Wang X, Huang Y, Guo Y, Zeng H (2021) Novel fluorescence immunoassay for the detection of zearalenone using HRP-mediated fluorescence quenching of gold-silver bimetallic nanoclusters. Food Chem 355:129633. https://doi.org/10.1016/j.foodchem.2021.129633
Bhunia S, Kumar S, Purkayastha P (2019) Dependence of ultrafast dynamics in gold–silver alloy nanoclusters on the proportion of the metal content. SN Appl Sci 1:449. https://doi.org/10.1007/s42452-019-0473-9
Ye C, Chen X, Xu J, Xi H, Wu T, Deng D, Zhang J, Huang G (2020) Highly sensitive detection to gallic acid by polypyrrole-based MIES supported by MOFs-Co2+ @Fe3O4. J Electroanal Chem 859:113839. https://doi.org/10.1016/j.jelechem.2020.113839
Singh A, Bajpai V, Kumar S, Sharma KR, Kumar B (2016) Profiling of gallic and ellagic acid derivatives in different plant parts of Terminalia arjuna by HPLC-ESI-QTOF-MS/MS. Nat Prod Commun 11:239–244
Wu M, Yin C, Jiang X, Sun Q, Xu X, Ma Y, Liu X, Niu N, Chen L (2022) Biocompatible abscisic acid-sensing supramolecular hybridization probe for spatiotemporal fluorescence imaging in plant tissues. Anal Chem 94:8999–9008. https://doi.org/10.1021/acs.analchem.2c01050
Bhardwaj S, Kapoor D (2021) Fascinating regulatory mechanism of silicon for alleviating drought stress in plants. Plant Physiol Biochem 166:1044–1053. https://doi.org/10.1016/j.plaphy.2021.07.005
Kyraleou M, Kallithraka S, Theodorou N, Teissedre P-L, Kotseridis Y, Koundouras S (2017) Changes in tannin composition of syrah grape skins and seeds during fruit ripening under contrasting water conditions. Molecules 22:1453. https://doi.org/10.3390/molecules22091453
Sirin S, Aslim B (2019) Determination of antioxidant capacity, phenolic acid composition and antiproliferative effect associated with phenylalanine ammonia lyase (PAL) activity in some plants naturally growing under salt stress. Med Chem Res 28:229–238. https://doi.org/10.1007/s00044-018-2278-6
Acknowledgements
We would like to acknowledge the technical support from Analysis and Testing Center of Northeast Forestry University.
Funding
This project was funded by the Fundamental Research Funds for the Central Universities (2572022DJ01), the Natural Science Foundation of Heilongjiang Province (LH2022B004), the 111 Project (B20088), the Heilongjiang Touyan Innovation Team Program (Tree Genetics and Breeding Innovation Team).
Author information
Authors and Affiliations
Contributions
MW: conceptualization, method, writing—review and editing. ZL: data curation, investigation. MW: method. TW: conceptualization. XY: conceptualization. NN: funding acquisition, writing—review and editing, supervision. LC: funding acquisition, writing—review and editing, supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, M., Liu, Z., Wu, M. et al. Ratiometric luminescent sensor based on BSA-coated gold/silver nanoclusters for the selective determination and spatiotemporal imaging of gallic acid in plants. Microchim Acta 191, 60 (2024). https://doi.org/10.1007/s00604-023-06156-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-06156-5