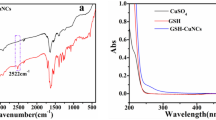
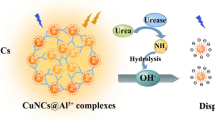
Abstract
Benefit from the strong coordination property, lanthanide metal ions have been used as competitive reagents to modulate the fluorescence changes of the system. However, lanthanide metal ions as inducers for aggregation-induced emission enhancement in nanosystems is rare. Herein, we report a “turn on–off-on” fluorescent switch for cascade detection of acid phosphatase (ACP) and adenosine triphosphate (ATP) based on the competitive coordination of samarium ions (Sm3+). Novel copper nanoclusters (CuNCs) with long wavelength emission (614 nm) stabilized by glutathione (GSH) and glycylglycine (Gly-Gly) have been confirmed to have AIE property. With the continuous aggregation of GSH/Gly-Gly CuNCs under the induction of Sm3+, the fluorescence of the system increased to achieve the “turn-on” process. The coordinated behaviour between Sm3+ and GSH/Gly-Gly CuNCs is discussed. Due to the strong metal coordination ability of ATP, the Sm3+ coordinated with the GSH/Gly-Gly CuNCs is competed out, resulting in the fluorescence “turn-off” process of the system. As the substrate of enzymatic hydrolysis of ACP, with the continuous hydrolysis of ATP by ACP, Sm3+ coordinates with GSH/Gly-Gly CuNCs again, which leads to the AIE effect and realize the fluorescence "turn-on" process of the system. This strategy results in ATP linear range of 0.508 ~ 120.0 μM with a detection limit of 0.508 μM (S/N = 3) and ACP linear range of 0.011 ~ 30.0 U·L−1 with a detection limit of 0.011 U·L−1 (S/N = 3). Application to biologic samples was successful.
Graphical Abstract








Similar content being viewed by others
Data availability
Data generated or analyzed during this study are included in this article and its supplementary file.
References
Kwok RTK, Leung CWT, Lam JWY, Tang BZ (2015) Biosensing by luminogens with aggregation-induced emission characteristics. Chem Soc Rev 44:4228–4238. https://doi.org/10.1039/C4CS00325J
Asad M, Anwar MI, Abbas A, Younas A, Hussain S, Gao R, Li L-K, Shahid M, Khan S (2022) AIE based luminescent porous materials as cutting-edge tool for environmental monitoring: State of the art advances and perspectives. Coord Chem Rev 463:214539. https://doi.org/10.1016/j.ccr.2022.214539
Wang D, Tang BZ (2019) Aggregation-induced emission luminogens for activity-based sensing. Acc Chem Res 52:2559–2570. https://doi.org/10.1021/acs.accounts.9b00305
Wu W, Liu B (2021) Aggregation-induced emission: challenges and opportunities. National ScienceReview 8:222. https://doi.org/10.1093/nsr/nwaa222
Liu Y, Chen X, Liu X, Guan W, Lu C (2023) Aggregation-induced emission-active micelles: synthesis, characterization, and applications. Chem Soc Rev 52:1456–1490. https://doi.org/10.1039/d2cs01021f
Prakash KT, Singh N, Venkatesh V (2019) Synthesis of novel luminescent copper nanoclusters with substituent driven self-assembly and aggregation induced emission (AIE). Chem Commun (Camb) 55:322–325. https://doi.org/10.1039/c8cc09109a
Shen J, Wen X, Fan Z (2023) Tandem detection of aluminum ion and norfloxacin by Au-doped copper nanoclusters based on AIE and coordination reaction. Sens Actuators, B Chem 381:133436. https://doi.org/10.1016/j.snb.2023.133436
Li X, Zhang X, Cao H, Huang Y, Feng P (2021) Tb3+ tuning AIE self-assembly of copper nanoclusters for sensitively sensing trace fluoride ions. Sens Actuators, B Chem 342:130071. https://doi.org/10.1016/j.snb.2021.130071
Geng F, Zou C, Liu J, Zhang Q, Guo X, Fan Y, Yu H, Yang S, Liu Z, Li L (2019) Development of luminescent nanoswitch for sensing of alkaline phosphatase in human serum based onAl3+-PPi interaction and Cu NCs with AIE properties. Anal Chim Acta 1076:131–137. https://doi.org/10.1016/j.aca.2019.05.026
Zhao M, Qian Z, Zhong M, Chen Z, Ao H, Feng H (2017) Fabrication of stable and luminescent copper nanocluster-based AIE particles and their application in β-galactosidase activity assay. ACS Appl Mater Interfaces 9:32887–32895. https://doi.org/10.1021/acsami.7b09659
Gao Y, Wei K, Li J, Li Y, Hu J (2018) A facile four-armed AIE fluorescent sensor for heparin and protamine. Sens Actuators, B Chem 277:408–414. https://doi.org/10.1016/j.snb.2018.09.054
Wang Z, Shi Y, Yang X, Xiong Y, Li Y, Chen B, Lai W-F, Rogach AL (2018) Water-soluble biocompatible copolymer hypromellose grafted chitosan able to load exogenous agents and copper nanoclusters with aggregation-induced emission. Adv Funct Mater 28:1802848. https://doi.org/10.1002/adfm.201802848
Huang X, Lan M, Wang J, Guo L, Lin Z, Sun N, Wu C, Qiu B (2020) A fluorescence signal amplification and specific energy transfer strategy for sensitive detection of β-galactosidase based on the effects of AIE and host-guest recognition. Biosens Bioelectron 169:112655. https://doi.org/10.1016/j.bios.2020.112655
Qu F, Yang Q, Wang B, You J (2020) Aggregation-induced emission of copper nanoclusters triggered by synergistic effect of dual metal ions and the application in the detection of H2O2 and related biomolecules. Talanta 207:120289. https://doi.org/10.1016/j.talanta.2019.120289
Liu J, Bao H, Ma D-L, Leung C-H (2018) Silver nanoclusters functionalized with Ce(III) ions are a viable “turn-on-off” fluorescent probe for sulfide. Mikrochim Acta 186:16. https://doi.org/10.1007/s00604-018-3149-z
Pan T, Zhou T, Tu Y, Yan J (2021) Turn-on fluorescence measurement of acid phosphatase activity through an aggregation-induced emission of thiolate-protected gold nanoclusters. Talanta 227:122197. https://doi.org/10.1016/j.talanta.2021.122197
Bull H, Murray PG, Thomas D, Fraser AM, Nelson PN (2002) Acid phosphatases. Mol0 Pathol MP 55:65–72. https://doi.org/10.1136/mp.55.2.65
Yin C, Wu M, Liu H, Sun Q, Sun X, Niu N, Xu J, Chen L (2022) Reversible AIE self-assembled nanohybrids coordinated by La3+ for ratiometric visual acid phosphatase monitoring and intracellular imaging. Sens Actuators, B Chem 371:132550. https://doi.org/10.1016/j.snb.2022.132550
Chen S, Chen X-B, Liu W-Y, Yu Y-L, Liu M-X (2023) Phosphorescence, fluorescence, and colorimetric triple-mode sensor for the detection of acid phosphatase and corresponding inhibitor. Anal Chim Acta 1275:341612. https://doi.org/10.1016/j.aca.2023.341612
Lin Z, Liu Z, Zhang H, Su X (2015) Near-infrared fluorescence probe for the determination of acid phosphatase and imaging of prostate cancer cells. Analyst 140:1629–1636. https://doi.org/10.1039/c4an01868k
Yan X, Xia C, Chen B, Li YF, Gao PF, Huang CZ (2020) Enzyme activity triggered blocking of plasmon resonance energy transfer for highly selective detection of acid phosphatase. Anal Chem 92:2130–2135. https://doi.org/10.1021/acs.analchem.9b04685
Liu J, Ye LY, Mo YY, Yang H (2022) Highly sensitive fluorescent quantification of acid phosphatase activity and its inhibitor pesticide Dufulin by a functional metal-organic framework nanosensor for environment assessment and food safety. Food Chem 370:131034. https://doi.org/10.1016/j.foodchem.2021.131034
Chen S, Li Z, Xue R, Huang Z, Jia Q (2022) Confining copper nanoclusters in three dimensional mesoporous silica particles: Fabrication of an enhanced emission platform for "turn off-on" detection of acid phosphatase activity. Anal Chim Acta 1192:339387. https://doi.org/10.1016/j.aca.2021.339387
Li S, Fu G, Wang Y, Xiang Y, Mu S, Xu Y, Liu X, Zhang H (2021) A dual-signal fluorescent probe for detection of acid phosphatase. Anal Bioanal Chem 413:3925–3932. https://doi.org/10.1007/s00216-021-03343-2
Ran F, Ma C, Xiang Y, Xu Y, Liu X, Zhang H (2021) A fluorescent and colorimetric dual-channel sensor based on acid phosphatase-triggered blocking of internal filtration effect. Mikrochim Acta 188:282. https://doi.org/10.1007/s00604-021-04951-6
Zhu Z, Lin X, Wu L, Zhao C, Li S, Liu A, Lin X, Lin L (2019) Nitrogen-doped carbon dots as a ratiometric fluorescent probe for determination of the activity of acid phosphatase, for inhibitor screening, and for intracellular imaging. Mikrochim Acta 186:558. https://doi.org/10.1007/s00604-019-3600-9
You J-G, Lu C-Y, Krishna Kumar AS, Tseng W-L (2018) Cerium(iii)-directed assembly of glutathione-capped gold nanoclusters for sensing and imaging of alkaline phosphatase-mediated hydrolysis of adenosine triphosphate. Nanoscale 10:17691–17698. https://doi.org/10.1039/C8NR05050C
Wang G, Su X, Xu Q, Xu G, Lin J, Luo X (2018) Antifouling aptasensor for the detection of adenosine triphosphate in biological media based on mixed self-assembled aptamer and zwitterionic peptide. Biosens Bioelectron 101:129–134. https://doi.org/10.1016/j.bios.2017.10.024
Singh VR, Singh PK (2020) A supramolecule based fluorescence turn-on and ratiometric sensor for ATP in aqueous solution. J Mater Chem B 8:1182–1190. https://doi.org/10.1039/C9TB02403D
Chu B, Wang A, Cheng L, Chen R, Shi H, Song B, Dong F, Wang H, He Y (2021) Ex vivo and in vivo fluorescence detection and imaging of adenosine triphosphate. J Nanobiotechnology 19:187. https://doi.org/10.1186/s12951-021-00930-4
Chen C, Yuan Q, Ni P, Jiang Y, Zhao Z, Lu Y (2018) Fluorescence assay for alkaline phosphatase based on ATP hydrolysis-triggered dissociation of cerium coordination polymer nanoparticles. Analyst 143:3821–3828. https://doi.org/10.1039/C8AN00787J
Thammajinno S, Buranachai C, Kanatharana P, Thavarungkul P, Thammakhet-Buranachai C (2022) A copper nanoclusters probe for dual detection of microalbumin and creatinine, Spectrochimica acta. Part A, Mol Biomol Spectrosc 270:120816. https://doi.org/10.1016/j.saa.2021.120816
Li W, Wang X, Jiang T, Ma X, Tian H (2020) One-pot synthesis of β-cyclodextrin modified Au nanoclusters with near-infrared emission. Chem Commun (Camb) 56:5580–5583. https://doi.org/10.1039/d0cc00713g
Qi D-Y, Wang C, Gao Y-C, Li H-W, Wu Y (2022) Heteroatom doping and supramolecular assembly promoted copper nanoclusters to be a stable & high fluorescence sensor for trace amounts of ATP determination. Sens Actuators, B Chem 358:131469. https://doi.org/10.1016/j.snb.2022.131469
Li X, Hao S, Wang Z, Zhao C, Wang Z (2022) Improving the electrical conductivity and electrochemical performance of LiMn2O4 by Sm gaseous penetration technology. Appl Surf Sci 599:153923. https://doi.org/10.1016/j.apsusc.2022.153923
Acknowledgements
The authors gratefully acknowledge the financial support of Young and Middle-aged Teacher Education Research Project of Fujian Province (JAT220256).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, X., Chen, H., Huang, R. et al. Adjustable luminescence copper nanoclusters nanoswitch based on competitive coordination of samarium ions for cascade detection of adenosine triphosphate and acid phosphatase activity. Microchim Acta 191, 54 (2024). https://doi.org/10.1007/s00604-023-06138-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-06138-7