Abstract
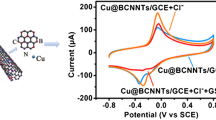
The present study selected 5, 5′-((6-(ethylamino)-1, 3, 5-triazine-2, 4-diyl) bis(azanediyl))diisophthalic acid (H4EATDIA) as ligand and an amino-functionalized cuprum-based MOF (EA-JUC-1000), successfully synthesized by microwave-assisted method, for proton conduction and dopamine sensing applications. In order to enhance the proton-conducting potential of EA-JUC-1000, the Brönsted acid (BA) encapsulated composites (BA@EA-JUC-1000) are dopped into chitosan (CS) to form a series of hybrid membranes (BA@EA-JUC-1000/CS). The impedance results display that the best proton conductivity of CF3SO3H@EA-JUC-1000/CS-8% reaches up to 1.23 × 10–3 S∙cm−1 at 338 K and ~ 98% RH, 2.6-fold than that of CS. Moreover, the EA-JUC-1000 is in-situ combined with reduced graphene oxide (rGO) (rGO/EA-JUC-1000), which makes EA-JUC-1000 have a wide detection range (0.1 ~ 500 μM) and a low limit of detection (50 nM), together with good anti-interference performance, reproducibility and repeatability. In addition, the electrochemical sensing method has been successfully applied to detect DA in bovine serum samples. The dual-functional MOF-based hybrid membrane and composites including proton conduction and DA sensing would provide an example of practical application for MOFs.
Graphical abstract







Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Zhu EB, Wu MH, Xu HZ, Peng BS, Liu ZY, Huang Y, Li YJ (2022) Stability of platinum-group-metal-based electrocatalysts in proton exchange membrane fuel cells. Adv Funct Mater 32:2203883. https://doi.org/10.1002/adfm.202203883
Cho J, Kim H, Oh H, Choi CH (2023) Elucidation of electrochemically induced but chemically driven Pt dissolution. JACS Au 3:105–112. https://doi.org/10.1021/jacsau.2c00474
Zhang X, Truong-Phuoc L, Asset T, Pronkin S, Pham-Huu C (2022) Are Fe–N–C electrocatalysts an alternative to Pt-based electrocatalysts for the next generation of proton exchange membrane fuel cells? ACS Catal 12:13853–13875. https://doi.org/10.1021/acscatal.2c02146
Liu X, Liang JS, Li Q (2023) Design principle and synthetic approach of intermetallic Pt-M alloy oxygen reduction catalysts for fuel cells. Chinese J Catal 45:17–26. https://doi.org/10.1016/s1872-2067(22)64165-2
Fan LH, Deng H, Zhang YG, Du Q, Leung DYC, Wang Y, Jiao K (2023) Towards ultralow platinum loading proton exchange membrane fuel cells. Energy Environ Sci 16:1466–1479. https://doi.org/10.1039/d2ee03169h
Ryu GY, Jae H, Kim KJ, Kim H, Lee S, Jeon Y, Roh D, Chi WS (2023) Hollow heteropoly acid-functionalized ZIF composite membrane for proton exchange membrane fuel cells. ACS Appl Energy Mater 6:4283–4296. https://doi.org/10.1021/acsaem.3c00220
Yang BL, Yu HF, Jia XD, Cheng Q, Ren YL, He B, Xiang ZH (2023) Atomically dispersed isolated Fe-Ce dual-metal-site catalysts for proton-exchange membrane fuel cells. ACS Appl Mater Interfaces 15:23316–23327. https://doi.org/10.1021/acsami.3c03203
Jiao K, Xuan J, Du Q, Bao ZM, Xie B, Wang BW, Zhao Y, Fan LH, Wang HZ, Hou ZJ, Huo S, Brandon NP, Yin Y, Guiver MD (2021) Designing the next generation of proton-exchange membrane fuel cells. Nature 595:361–369. https://doi.org/10.1038/s41586-021-03482-7
Jin HQ, Zou SY, Wen QL, Li YL, Ning FD, Xu PP, Pan SF, Zhou XC (2023) Performance improvement of air-breathing proton exchange membrane fuel cell (PEMFC) with a condensing-tower-like curved flow field. Chin Chem Lett 34:107441. https://doi.org/10.1016/j.cclet.2022.04.039
Zhu T, Yang YR, Liu YH, Lopez-Hallman R, Ma ZH, Liu L, Gong X (2020) Wireless portable light-weight self-charging power packs by perovskite-organic tandem solar cells integrated with solid-state asymmetric supercapacitors. Nano Energy 78:105397. https://doi.org/10.1016/j.nanoen.2020.105397
Zhu LJ, Wang SF, Sui PC, Gao X (2021) Multiscale modeling of an angled gas diffusion layer for polymer electrolyte membrane fuel cells: performance enhancing for aviation applications. Int J Hydrog Energy 46:20702–20714. https://doi.org/10.1016/j.ijhydene.2021.03.166
Pan MZ, Pan CJ, Li C, Zhao J (2021) A review of membranes in proton exchange membrane fuel cells: transport phenomena, performance and durability. Renew Sust Energ Rev 141:110771. https://doi.org/10.1016/j.rser.2021.110771
Min K, Al Munsur AZ, Paek SY, Jeon S, Lee SY, Kim TH (2023) Development of high-performance polymer electrolyte membranes through the application of quantum dot coatings to nafion membranes. ACS Appl Mater Interfaces 15:15616–15624. https://doi.org/10.1021/acsami.3c01289
Liu JM, Yu LW, Cai XK, Khan U, Cai ZY, Xi JY, Liu BL, Kang FY (2019) Sandwiching h-BN monolayer films between sulfonated poly(ether ether ketone) and nafion for proton exchange membranes with improved ion selectivity. ACS Nano 13:2094–2102. https://doi.org/10.1021/acsnano.8b08680
Ye JY, Yuan D, Ding M, Long Y, Long T, Sun LD, Jia CK (2021) A cost-effective nafion/lignin composite membrane with low vanadium ion permeation for high performance vanadium redox flow battery. J Power Source 482:229023. https://doi.org/10.1016/j.jpowsour.2020.229023
Kaur H, Siwal SS, Saini RV, Singh N, Thakur VK (2023) Significance of an electrochemical sensor and nanocomposites: toward the electrocatalytic detection of neurotransmitters and their importance within the physiological system. ACS Nanosci Au 3:1–27. https://doi.org/10.1021/acsnanoscienceau.2c00039
Cui M, Xin PC, Che ZM, Zou M, Zhang MR, Sun XF, Yuan YN, Zou ZH, Lv GQ, Wang S, Hu W (2023) Highly anti-interference electrocatalytic sensing for dopamine with nitrogen-doped graphdiyne directly in biofluids. Chem Eng J 464:142629. https://doi.org/10.1016/j.cej.2023.142629
Zhang JM, Ma YY, Han YH, Xu KZ, Yao S, Shi L, Zhu M (2023) 3D porous structure assembled from MXene via breath figure method for electrochemical detection of dopamine. Chem Eng J 452:139414. https://doi.org/10.1016/j.cej.2022.139414
Li RM, Guo WW, Zhu ZJ, Zhai YL, Wang GW, Liu Z, Jiao L, Zhu CZ, Lu XQ (2023) Single-atom indium boosts electrochemical dopamine sensing. Anal Chem 95:7195–7201. https://doi.org/10.1021/acs.analchem.2c05679
Sun XJ, Zhang L, Zhang XH, Liu XX, Jian J, Kong DC, Zeng DC, Yuan HM, Feng SH (2020) Electrochemical dopamine sensor based on superionic conducting potassium ferrite. Biosens Bioelectron 153:112045. https://doi.org/10.1016/j.bios.2020.112045
Kaya HK, Cinar S, Altundal G, Bayramlı Y, Unaleroglu C, Kuralay F (2021) A novel design thia-bilane structure-based molecular imprinted electrochemical sensor for sensitive and selective dopamine determination. Sens Actuators B Chem. 346:130425. https://doi.org/10.1016/j.snb.2021.130425
Dong YH, Xing J, Zhao T, Peng SG (2023) In situ growth of MIL-101(Cr)-SO3H sphere on polyethylenimine-reduced graphene oxide and its application as dopamine sensor. Diam Relat Mater 139:110304. https://doi.org/10.1016/j.diamond.2023.110304
Wang HY, Xie AJ, Li SJ, Wang JJ, Chen KX, Su ZL, Song NN, Luo SP (2022) Three-dimensional g-C3N4/MWNTs/GO hybrid electrode as electrochemical sensor for simultaneous determination of ascorbic acid, dopamine and uric acid. Anal Chim Acta 1211:339907. https://doi.org/10.1016/j.aca.2022.339907
You Y, Zou J, Li WJ, Chen J, Jiang XY, Yu JG (2022) Novel lanthanum vanadate-based nanocomposite for simultaneously electrochemical detection of dopamine and uric acid in fetal bovine serum. Int J Biol Macromol 195:346–355. https://doi.org/10.1016/j.ijbiomac.2021.12.058
Wang XM, Ma Y, Zhou Z, Zhang Z, Zhang J, Fan L, Du XZ, Lu XQ (2023) Ultrarapid synthesis of dumbbell-shaped carbon black-doped Ce (III, IV)-MOF composites for fabrication of simultaneous electrochemical sensor of dopamine and acetaminophen. Microchem J 195:109430. https://doi.org/10.1016/j.microc.2023.109430
Zhao DF, Li XJ, Zhang KY, Guo JZ, Huang XB, Wang G (2023) Recent advances in thermocatalytic hydrogenation of unsaturated organic compounds with metal-organic frameworks-based materials: construction strategies and related mechanisms. Coord Chem Rev 487:215159. https://doi.org/10.1016/j.ccr.2023.215159
Wang F, Gao Y, Liu SS, Yi XH, Wang CC, Fu H (2023) Fabrication strategies of metal–organic frameworks derivatives for catalytic aqueous pollutants elimination. Chem Eng J 463:142466. https://doi.org/10.1016/j.cej.2023.142466
Xu HK, Wei XF, Zeng H, Jiang CH, Wang ZF, Ouyang YG, Lu CY, Jing Y, Yao SW, Dai FN (2023) Recent progress of two-dimensional metal-organic-frameworks: from synthesis to electrocatalytic oxygen evolution. Nano Res 16:8614–8637. https://doi.org/10.1007/s12274-023-5576-3
Widera A, Thony D, Aebli M, Oppenheim JJ, Andrews JL, Eiler F, Worle M, Schonberg H, Weferling N, Dinca M, Grutzmacher H (2023) Solid-state investigation, storage, and separation of pyrophoric PH3 and P2H4 with α-Mg formate. Angew Chem Int Ed Engl 62:e202217534. https://doi.org/10.1002/anie.202217534
Pachfule P, Balan BK, Kurungot S, Banerjee R (2012) One-dimensional confinement of a nanosized metal organic framework in carbon nanofibers for improved gas adsorption. Chem Commun 48:2009–2011. https://doi.org/10.1039/c2cc16877d
Suresh K, Matzger AJ (2019) Enhanced drug delivery by dissolution of amorphous drug encapsulated in a water unstable metal-organic framework (MOF). Angew Chem Int Ed Engl 58:16790–16794. https://doi.org/10.1002/anie.201907652
Wu MX, Yang YW (2017) Metal-organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv Mater 29:1606134. https://doi.org/10.1002/adma.201606134
Kasula M, Le T, Thomsen A, Esfahani MR (2022) Silver metal organic frameworks and copper metal organic frameworks immobilized on graphene oxide for enhanced adsorption in water treatment. Chem Eng J 439:135542. https://doi.org/10.1016/j.cej.2022.135542
Lou XX, Zhi FK, Sun XY, Wang F, Hou XH, Lv CN, Hu Q (2023) Construction of co-immobilized laccase and mediator based on MOFs membrane for enhancing organic pollutants removal. Chem Eng J 451:138080. https://doi.org/10.1016/j.cej.2022.138080
Yang J, Lin J, Sun SQ, Li X, Liu L, Wang C (2023) Multidimensional network of polypyrrole nanotubes loaded with ZIF-67 to construct multiple proton transport channels in composite proton exchange membranes for fuel cells. J Mater Sci Technol 152:75–85. https://doi.org/10.1016/j.jmst.2022.11.063
Xiao L, Xia YS, Yu Y, Cao HY, Lu YQ, Zhang DZ, Huang K, Xu Z (2023) Usability of unstable metal organic framework enabled by carbonization within flow battery membrane under harsh environment. J Membr Sci 671:121349. https://doi.org/10.1016/j.memsci.2023.121349
Ke XY, Zhao Z, Huang JY, Liu C, Huang GS, Tan J, Zhu HQ, Xiao ZJ, Liu XY, Mei YF, Chu JH (2023) Growth control of metal-organic framework films on marine biological carbon and their potential-dependent dopamine sensing. ACS Appl Mater Interfaces 15:12005–12016. https://doi.org/10.1021/acsami.2c20517
Shu Y, Yan Y, Chen JY, Xu Q, Pang H, Hu XY (2017) Ni and NiO nanoparticles decorated metal-organic framework nanosheets: facile synthesis and high-performance nonenzymatic glucose detection in human serum. ACS Appl Mater Interfaces 9:22342–22349. https://doi.org/10.1021/acsami.7b07501
Xie YQ, Zong SW, Lu L, Zhang KL (2022) Proton conduction and electrochemical enzyme-free glucose sensitive sensing based on a newly constructed Co-MOF and its composite with hydroxyl carbon nanotubes. Polyhedron 226:116095. https://doi.org/10.1016/j.poly.2022.116095
Zhou SF, Hao BB, Lin T, Zhang CX, Wang QL (2020) A dual-functional MOF for high proton conduction and sensitive detection of ascorbic acid. Dalton Trans 49:14490–14496. https://doi.org/10.1039/d0dt02834g
Chen X, Li G (2020) Proton conductive Zr-based MOFs. Inorg Chem Front 7:3765–3784. https://doi.org/10.1039/d0qi00883d
Liu YR, Chen YY, Zhuang Q, Li G (2022) Recent advances in MOFs-based proton exchange membranes. Coord Chem Rev 471:214740. https://doi.org/10.1016/j.ccr.2022.214740
Yoo DK, Lee G, Mondol MMH, Lee HJ, Kim CM, Jhung SH (2023) Preparation and applications of metal–organic frameworks composed of sulfonic acid. Coord Chem Rev 474:214868. https://doi.org/10.1016/j.ccr.2022.214868
Chen J, Mei QQ, Chen YL, Marsh C, An B, Han X, Silverwood IP, Li M, Cheng YQ, He M, Chen X, Li WY, Kippax-Jones M, Crawshaw D, Frogley MD, Day SJ, Garcia-Sakai V, Manuel P, Ramirez-Cuesta AJ, Yang SH, Schroder M (2022) Highly efficient proton conduction in the metal-organic framework material MFM-300(Cr)·SO4(H3O)2. J Am Chem Soc 144:11969–11974. https://doi.org/10.1021/jacs.2c04900
Zhang J, He X, Kong YR, Luo HB, Liu M, Liu Y, Ren XM (2021) Efficiently boosting moisture retention capacity of porous superprotonic conducting MOF-802 at ambient humidity via forming a hydrogel composite strategy. ACS Appl Mater Interfaces 13:37231–37238. https://doi.org/10.1021/acsami.1c11054
Yang Y, He XY, Zhang PH, Andaloussi YH, Zhang HL, Jiang ZY, Chen Y, Ma SQ, Cheng P, Zhang ZJ (2020) Combined intrinsic and extrinsic proton conduction in robust covalent organic frameworks for hydrogen fuel cell applications. Angew Chem Int Ed Engl 59:3678–3684. https://doi.org/10.1002/anie.201913802
Sahoo R, Mondal S, Pal SC, Mukherjee D, Das MC (2021) Covalent-organic frameworks (COFs) as proton conductors. Adv Energy Mater 11:2102300. https://doi.org/10.1002/aenm.202102300
Shi RM, Zhang ZQ, Yang F, Zhong CL (2022) Simultaneously anchoring free carboxyl and sulfonate groups into a metal-organic framework for high proton conductivity. Microporous Mesoporous Mater 343:112192. https://doi.org/10.1016/j.micromeso.2022.112192
Ren HM, Wang HW, Jiang YF, Tao ZX, Mu CY, Li G (2022) Proton conductive lanthanide-based metal-organic frameworks: synthesis strategies, structural features, and recent progress. Top Curr Chem 380:9. https://doi.org/10.1007/s41061-022-00367-9
Zhao SN, Zhang Y, Song SY, Zhang HJ (2019) Design strategies and applications of charged metal organic frameworks. Coord Chem Rev 398:113007. https://doi.org/10.1016/j.ccr.2019.07.004
Paul J, Kim J (2023) Reticular synthesis of a conductive composite derived from metal-organic framework and Mxene for the electrochemical detection of dopamine. Appl Surf Sci 613:156103. https://doi.org/10.1016/j.apsusc.2022.156103
Chang YN, Shen CH, Huang CW, Tsai MD, Kung CW (2023) Defective metal–organic framework nanocrystals as signal amplifiers for electrochemical dopamine sensing. ACS Appl Nano Mater 6:3675–3684. https://doi.org/10.1021/acsanm.2c05402
Shi XF, Xu YL, Zhao B, Li PP, Song M, Jia JJ, Yu HJ, Lu G (2021) Integrating conductive metal–organic framework with graphene oxide to highly sensitive platform for electrochemical sensing. Adv. Mater. 8:2100586. https://doi.org/10.1002/admi.202100586
Bai HJ, Zhang HQ, He YK, Liu JD, Zhang B, Wang JT (2014) Enhanced proton conduction of chitosan membrane enabled by halloysite nanotubes bearing sulfonate polyelectrolyte brushes. J Membr Sci 454:220–232. https://doi.org/10.1016/j.memsci.2013.12.005
Kreuer KD, Rabenau A, Weppner W (1982) Vehicle mechanism, a new model for the interpretation of the conductivity of fast proton conductors. Angew Chem Int Ed Engl 21:208–209. https://doi.org/10.1002/anie.198202082
Agmon N (1995) The grotthuss mechanism. Chem Phys Lett 244:456–462. https://doi.org/10.1016/0009-2614(95)00905-J
Acknowledgements
The authors acknowledge financial support from the Natural Science Foundation of Heilongjiang Province, China (Nos. YQ2020E029 and TD2020B001), and National Natural Science Foundation of China (Nos. 21771047 and 52072100).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, M., Tan, W., Wu, X. et al. A dual-functional cuprum coordination framework for high proton conduction and electrochemical dopamine detection. Microchim Acta 191, 67 (2024). https://doi.org/10.1007/s00604-023-06133-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-06133-y