Abstract
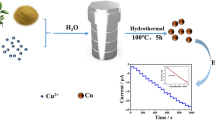
Carbon-coated copper nanocrystals (CuNCs) with peroxidase-like activity were hydrothermally prepared by using copper acetate, citric acid (CA) and histidine (His) as the precursors. Various shaped CuNCs, including urchin-like, slab-like and spherical appearance were facilely prepared by addition of different amount of NaNO2 in the precursor solutions. When 3,3′,5,5′-tetramethylbenzidine (TMB) was used as the substrate, the CuNCs with urchin-like appearance have greatest peroxidase-like activity and their Michaelis–Menten constant (Km) and the maximum rate constant (νmax) are respectively 8.8 and 1.2 times higher than that obtained from horseradish peroxidase (HRP). The production of reactive oxygen species (ROS) was confirmed by radical quenching and electron spin resonance (ESR) tests. Subsequent studies have found that the CuNCs catalyzed color reaction of TMB can be selectively quenched by the environmental pollutant 2,4-dinitrophenylhydrazine (2,4-DNPH). Thus a new colorimetric method for the determination of 2,4-DNPH with a linear range of 0.60–20 µM was developed and a limit of detection (LOD) as low as 0.166 µM was achieved. The results obtained not only reveal the tunability of the peroxidase-like activity of Cu-based nanomaterials, but also provide a new method for the sensitive determination of environmental contaminate.
Graphical abstract







Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the fundings of this study are available within the article [and/or its supplementatry materials].
References
Fedor MJ, Williamson JR (2005) The catalytic diversity of RNAS, 1. Nat Rev Mol Cell Biol 6(5):399–412. https://doi.org/10.1038/nrm1647
Dawson JH (1988) Probing structure-function relations in heme-containing oxygenases and peroxidases. Science (New York, N.Y.) 240(4851):433–439. https://doi.org/10.1126/science.3358128
Huang Y, Ren J, Qu X (2019) Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem Rev 119(6):4357–4412. https://doi.org/10.1021/acs.chemrev.8b00672
Ge C, Wu R, Chong Y, Fang G, Jiang X, Pan Y, Chen C, Yin J-J (2018) Synthesis of Pt hollow nanodendrites with enhanced peroxidase-Like activity against bacterial infections: implication for wound healing. Adv Funct Mater 28(28):1801484. https://doi.org/10.1002/adfm.201801484
Xin Q, Jia X, Nawaz A, Xie W, Li L, Gong JR (2020) Mimicking peroxidase active site microenvironment by functionalized graphene quantum dots. Nano Res 13(5):1427–1433. https://doi.org/10.1007/s12274-020-2678-z
Chen L, Sun B, Wang X, Qiao F, Ai S (2013) 2D ultrathin nanosheets of Co-Al layered double hydroxides prepared in L-asparagine solution: enhanced peroxidase-like activity and colorimetric detection of glucose. J Mater Chem B 1(17):2268–2274. https://doi.org/10.1039/c3tb00044c
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2(9):577–583. https://doi.org/10.1038/nnano.2007.260
Liu Q, Zhang A, Wang R, Zhang Q, Cui D (2021) A Review on metal- and metal oxide-based nanozymes: properties, mechanisms, and applications. Nano-Micro Lett 13(1):154. https://doi.org/10.1007/s40820-021-00674-8
Lyu Z, Shang Y, Xia Y (2022) Shape-controlled synthesis of copper nanocrystals for plasmonic, biomedical, and electrocatalytic applications. Acc Mater Res 3(11):1137–1148. https://doi.org/10.1021/accountsmr.2c00134
Brillas E, Banos MA, Camps S, Arias C, Cabot PL, Garrido JA, Rodriguez RM (2004) Catalytic effect of Fe2+, Cu2+ and UVA light on the electrochemical degradation of nitrobenzene using an oxygen-diffusion cathode. New J Chem 28(2):314–322. https://doi.org/10.1039/b312445b
Ma B, Wang S, Liu F, Zhang S, Duan J, Li Z, Kong Y, Sang Y, Liu H, Bu W, Li L (2019) Self-assembled copper-amino acid nanoparticles for in situ glutathione “AND” H2O2 sequentially triggered chemodynamic therapy. J Am Chem Soc 141(2):849–857. https://doi.org/10.1021/jacs.8b08714
Xi J, Wei G, An L, Xu Z, Xu Z, Fan L, Gao L (2020) Copper/carbon hybrid nanozyme: tuning catalytic activity by the copper state for antibacterial therapy (vol 19, pg 7645, 2019). Nano Lett 20(1):800–800. https://doi.org/10.1021/acs.nanolett.9b02242
Liu T, Xiao B, Xiang F, Tan J, Chen Z, Zhang X, Wu C, Mao Z, Luo G, Chen X, Deng J (2020) Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat Commun 11(1):2788. https://doi.org/10.1038/s41467-020-16544-7
Zhang Z, Wang S-S, Song R, Cao T, Luo L, Chen X, Gao Y, Lu J, Li W-X, Huang W (2017) The most active Cu facet for low-temperature water gas shift reaction. Nat Commun 8:488. https://doi.org/10.1038/s41467-017-00620-6
Kim D, Kley CS, Li Y, Yang P (2017) Copper nanoparticle ensembles for selective electroreduction of CO2 to C-2-C-3 products. Proc Natl Acad Sci U S A 114(40):10560–10565. https://doi.org/10.1073/pnas.1711493114
Pileni MP, Ninham BW, Gulik-Krzywicki T, Tanori J, Lisiecki I, Filankembo A (1999) Direct relationship between shape and size of template and synthesis of copper metal particles. Adv Mater 11(16):1358–1362. https://doi.org/10.1016/j.jcrysgro.2019.05.028
Joshi SS, Patil SF, Iyer V, Mahumuni S (1998) Radiation induced synthesis and characterization of copper nanoparticles. Nanostruct Mater 10(7):1135–1144. https://doi.org/10.1016/S0965-9773(98)00153-6
Su X, Zhao J, Bala H, Zhu Y, Gao Y, Ma S, Wang Z (2007) Fast synthesis of stable cubic copper nanocages in the aqueous phase. J Phys Chem C 111(40):14689–14693. https://doi.org/10.1021/jp074550w
Xie SY, Ma ZJ, Wang CF, Lin SC, Jiang ZY, Huang RB, Zheng LS (2004) Preparation and self-assembly of copper nanoparticles via discharge of copper rod electrodes in a surfactant solution: a combination of physical and chemical processes. J Solid State Chem 177(10):3743–3747. https://doi.org/10.1016/j.jssc.2004.07.012
Hu Y, He Y, Han Y, Ge Y, Song G, Zhou J (2018) Determination of the activity of alkaline phosphatase based on aggregation-induced quenching of the fluorescence of copper nanoclusters. Microchim Acta 186(1):5. https://doi.org/10.1007/s00604-018-3122-x
Lee S-W, Kim M-S, Jeong JH, Kim D-H, Chung KY, Roh KC, Kim K-B (2017) Li3PO4 surface coating on Ni-rich LiNi0.6Co0.2Mn0.2O2 by a citric acid assisted sol-gel method: Improved thermal stability and high-voltage performance. J Power Sources 360:206–214. https://doi.org/10.1016/j.jpowsour.2017.05.042
Behdadfar B, Kermanpur A, Sadeghi-Aliabadi H, del Puerto Morales M, Mozaffari M (2012) Synthesis of high intrinsic loss power aqueous ferrofluids of iron oxide nanoparticles by citric acid-assisted hydrothermal-reduction route. J Solid State Chem 187:20–26. https://doi.org/10.1016/j.jssc.2011.12.011
Ragavan KV, Rastogi NK (2016) Graphene-copper oxide nanocomposite with intrinsic peroxidase activity for enhancement of chemiluminescence signals and its application for detection of Bisphenol-A. Sens Actuators B 229:570–580. https://doi.org/10.1016/j.snb.2016.02.017
Yang D, Li Q, Tammina SK, Gao Z, Yang Y (2020) Cu-CDs/H2O2 system with peroxidase-like activities at neutral pH for the co-catalytic oxidation of o-phenylenediamine and inhibition of catalytic activity by Cr(III). Sens Actuators B 319:128273. https://doi.org/10.1016/j.snb.2020.128273
Herricks T, Chen J, Xia Y (2004) Polyol synthesis of platinum nanoparticles: control of morphology with sodium nitrate. Nano Lett 4(12):2367–2371. https://doi.org/10.1021/nl048570a
Garcia-Alonso S, Maria Bernal-Paez A, Maria Perez-Pastor R (2021) Reduced solvent and reagent amounts: effect on carbonyl dinitrophenylhydrazone measurements at low concentrations. Anal Methods 13(16):1976–1985. https://doi.org/10.1039/d0ay02288h
Malaei R, Ramezani AM, Absalan G (2018) Analysis of malondialdehyde in human plasma samples through derivatization with 2,4-dinitrophenylhydrazine by ultrasound-assisted dispersive liquid-liquid microextraction-GC-FID approach. Anal Methods 1089:60–69. https://doi.org/10.1016/j.jchromb.2018.05.001
Zhang E, Ju P, Zhang Z, Yang H, Tang L, Hou X, You J, Wang J-J (2019) A novel multi-purpose Zn-MOF fluorescent sensor for 2,4-dinitrophenylhydrazine, picric acid, La3+ and Ca2+: Synthesis, structure, selectivity, sensitivity and recyclability. Spectrochim Acta Part A 222:117207. https://doi.org/10.1016/j.saa.2019.117207
Jiao X, Marin L, Cheng X (2022) Fluorescent cellulose/testing paper for the sensitive and selective recognition of explosives 2,4,6-trinitrophenol and 2,4-dinitrophenylhydrazine. J Photochem Photobiol A 424:113632. https://doi.org/10.1016/j.jphotochem.2021.113632
Koyappayil A, Kim HT, Lee MH (2021) ‘Laccase-like’ properties of coral-like silver citrate micro-structures for the degradation and determination of phenolic pollutants and adrenaline. J Hazard Mater 412:125211. https://doi.org/10.1016/j.jhazmat.2021.125211
Zuo YG, Deng YW (1998) The near-UV absorption constants for nitrite ion in aqueous solution. Chemosphere 36(1):181–188. https://doi.org/10.1016/S0045-6535(97)10028-5
Lu W, Yuan M, Chen J, Zhang J, Kong L, Feng Z, Ma X, Su J, Zhan J (2021) Synergistic Lewis acid-base sites of ultrathin porous Co3O4 nanosheets with enhanced peroxidase-like activity. Nano Res 14(10):3514–3522. https://doi.org/10.1007/s12274-021-3656-9
Wang J, Huang R, Qi W, Su R, He Z (2022) Preparation of amorphous MOF based biomimetic nanozyme with high laccase- and catecholase-like activity for the degradation and detection of phenolic compounds. Chem Eng J 434:134677. https://doi.org/10.1016/j.cej.2022.134677
Yang L, Li N, Guo C, He J, Wang S, Qiao L, Li F, Yu L, Wang M, Xu X (2021) Marine biomass-derived composite aerogels for efficient and durable solar-driven interfacial evaporation and desalination. Chem Eng J 417:128051. https://doi.org/10.1016/j.cej.2020.128051
Zhong Y, Liao P, Kang J, Liu Q, Wang S, Li S, Liu X, Li G (2023) Locking effect in metal@MOF with superior stability for highly chemoselective catalysis. J Am Chem Soc 145(8):4659–4666. https://doi.org/10.1021/jacs.2c12590
Jin G, Liu J, Wang C, Gu W, Ran G, Liu B, Song Q (2020) Ir nanoparticles with multi-enzyme activities and its application in the selective oxidation of aromatic alcohols. Appl Catal B 267:118725. https://doi.org/10.1016/j.apcatb.2020.118725
Rahman MZ, Tapping PC, Kee TW, Smernik R, Spooner N, Moffatt J, Tang Y, Davey K, Qiao SZ (2017) A benchmark quantum yield for water photoreduction on amorphous carbon nitride. Adv Funct Mater 27(39):1702384. https://doi.org/10.1002/adfm.201702384
Zhao G, Liu S, Zhang X, Zhang Y, Shi H, Liu Y, Hou L, Yuan C (2023) Construction of Co@N-CNTs grown on N-MoxC nanosheets for separator modification to enhance adsorption and catalytic conversion of polysulfides in Li–S batteries. J Mater Chem A 11(4):1856–1865. https://doi.org/10.1039/d2ta09123b
Wang J, Huang R, Qi W, Su R, He Z (2022) Construction of biomimetic nanozyme with high laccase- and catecholase-like activity for oxidation and detection of phenolic compounds. J Hazard Mater 429:128404. https://doi.org/10.1016/j.jhazmat.2022.128404
Liu J, Wang X, Ma F, Yang X, Liu Y, Zhang X, Guo S, Wang Z, Yang S, Zhao R (2022) Atomic copper(I)-carbon nitride as a peroxidase-mimic catalyst for high selective detection of perfluorooctane sulfonate. Chem Eng J 435:134966. https://doi.org/10.1016/j.cej.2022.134966
Lei L, Song D, Fan L, Liu B, He M, Sun X, Xu W, Tao K, Huang H, Li Y (2022) Determination of catechin and glutathione using copper aspartate nanofibers with multiple enzyme-like activities. Microchim Acta 189(2):61. https://doi.org/10.1007/s00604-021-05160-x
Hu L, Yuan Y, Zhang L, Zhao J, Majeed S, Xu G (2013) Copper nanoclusters as peroxidase mimetics and their applications to H2O2 and glucose detection. Anal Chim Acta 762:83–86. https://doi.org/10.1016/j.aca.2012.11.056
Yang S, Yang X, Shao X, Niu R, Wang L (2011) Activated carbon catalyzed persulfate oxidation of Azo dye acid orange 7 at ambient temperature. J Hazard Mater 186(1):659–666. https://doi.org/10.1016/j.jhazmat.2010.11.057
Shen B, Dong C, Ji J, Xing M, Zhang J (2019) Efficient Fe(III)/Fe(II) cycling triggered by MoO2 in Fenton reaction for the degradation of dye molecules and the reduction of Cr(VI). Chin Chem Lett 30(12):2205–2210. https://doi.org/10.1016/j.cclet.2019.09.052
Dou J, Cheng J, Lu Z, Tian Z, Xu J, He Y (2022) Biochar co-doped with nitrogen and boron switching the free radical based peroxydisulfate activation into the electron-transfer dominated nonradical process. Appl Catal B 301:120832. https://doi.org/10.1016/j.apcatb.2021.120832
Wang D, Marin L, Cheng X (2022) Chitosan-bodipy macromolecular fluorescent probes prepared by click reactions for highly sensitive and selective recognition of 2,4-dinitrophenylhydrazine. New J Chem 46(43):20699–20710. https://doi.org/10.1039/d2nj03923k
Dong S, Lian X, Chen S, Li H, Liu E, Xu K (2021) Kinetic analysis and mechanism study on the photocatalytic degradation of 2,4-dinitrophenylhydrazine over surface plasmonic Ag/Cu/TiO2 composite. React Kinet Mech Catal 134(1):485–499. https://doi.org/10.1007/s11144-021-02054-0
Funding
This work was supported by Fundamental Research Funds for the Central Universities (JUSRP22027). We would like to acknowledge the work of Central Laboratory, School of Chemical and Material Engineering, Jiangnan University.
Author information
Authors and Affiliations
Contributions
Zhanghong Guo: Methodology, experiments and writing-original draft. Lin Zhou: Results validation. Xuan Chen: Results validation. Qijun Song: Methodology, writing-review & editing, supervision, funding acquisition.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, Z., Zhou, L., Chen, X. et al. Carbon-coated copper nanocrystals with enhanced peroxidase-like activity for sensitive colorimetric determination of 2,4-dinitrophenylhydrazine. Microchim Acta 191, 37 (2024). https://doi.org/10.1007/s00604-023-06127-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-06127-w