Abstract
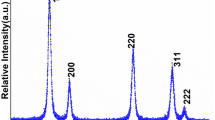
Research into metal nanoparticles has increased, and they have been increasingly used in non-enzymatic sensor applications. However, there have been few reports on the use of natural products to prepare non-precious metal nanoparticles in an eco-friendly (green) manner. Studies were conducted on Cu nanoparticles (Cu NPs) prepared by the use of natural gallic acid (GA). This method is environmentally friendly and can be applied to H2O2 detection. As-obtained Cu NPs were characterized by x-ray photoelectron spectroscopy (XPS), powder x-ray diffraction (XRD), and transmission electron microscopy (TEM). When applied to H2O2 detection, Cu NPs had good sensitivity, stability, and anti-interference properties. Its electrochemical performance is no less than that of precious metal nanomaterials. Cu NPs mainly exhibit good sensitivity, reaching 5.74 μA mM-1 cm-2; the concentration range thereof is 1–20 mM. The process of constructing nanoparticles in this work used natural products, making it environmentally friendly and proving the practicality of constructing non-precious metal nanoparticles and the feasibility of their application to H2O2 detection.
Graphic Abstract







Similar content being viewed by others
References
Y. Gao, F. Yang, Q. Yu, R. Fan, M. Yang, S. Rao, Q. Lan, and Z. Yang, Mikrochim. Acta. 186, 192 (2019). https://doi.org/10.1007/s00604-019-3263-6.
S. Daemi, S. Ghasemi, and A. Akbar Ashkarran, J. Colloid Interface Sci. 550, 180 (2019). https://doi.org/10.1016/j.jcis.2019.04.091.
X. Wu, F. Li, C. Zhao, and X. Qian, Sensors Actuat. B Chem. 274, 163 (2018). https://doi.org/10.1016/j.snb.2018.07.141.
D. Cheng, T. Wang, G. Zhang, H. Wu, and H. Mei, J. Alloys Compounds. 819, 153014 (2020). https://doi.org/10.1016/j.jallcom.2019.153014.
B.J. Brownlee, M. Bahari, J.N. Harb, J.C. Claussen, and B.D. Iverson, ACS Appl. Mater. Interfaces. 10, 28351 (2018). https://doi.org/10.1021/acsami.8b08997.
Y. Ren, F.Y. Wang, Z.J. Chen, R.T. Lan, R.H. Huang, W.Q. Fu, R.M. Gul, J. Wang, J.Z. Xu, and Z.M. Li, J. Mater. Chem. B. 8, 10428 (2020). https://doi.org/10.1039/d0tb01677b.
T. Dayakar, K.V. Rao, K. Bikshalu, V. Malapati, and K.K. Sadasivuni, Biosens Bioelectron. 111, 166 (2018). https://doi.org/10.1016/j.bios.2018.03.063.
Y. Su, H. Guo, Z. Wang, Y. Long, W. Li, and Y. Tu, Sens. Actuat. B Chem. 255, 2510 (2018). https://doi.org/10.1016/j.snb.2017.09.056.
D. Yin, X. Bo, J. Liu, and L. Guo, Anal. Chim. Acta 1038, 11–20 (2018). https://doi.org/10.1016/j.aca.2018.06.086.
S. Cheng, S. DelaCruz, C. Chen, Z. Tang, T. Shi, C. Carraro, and R. Maboudian, Sens. Actuat. B Chem. 298, 126860 (2019). https://doi.org/10.1016/j.snb.2019.126860.
X. Han, X. Wu, Y. Deng, J. Liu, J. Lu, C. Zhong, and W. Hu, Adv. Energy Mater. 8, 1800935 (2018). https://doi.org/10.1002/aenm.201870110.
Y. Zhang, Q. Wang, D. Liu, Q. Wang, T. Li, and Z. Wang, Appl. Surf. Sci. 521, 146434 (2020). https://doi.org/10.1016/j.apsusc.2020.146434.
C. Yuan, B.W. Hao, Y. Xie, and X.W. Lou, Angewandte Chemie International ed. in English. 53, 1488 (2014). https://doi.org/10.1002/anie.201303971.
K. Bindu, and H.S. Nagaraja, Mater. Res. Exp. 6, 095015 (2019). https://doi.org/10.1088/2053-1591/ab2ca8.
M.S. Garud, and Y.A. Kulkarni, Chem. Biol. Interact. 282, 69 (2018). https://doi.org/10.1016/j.cbi.2018.01.010.
A. Aydogdu, G. Sumnu, and S. Sahin, Carbohyd. Polym. 208, 241 (2019). https://doi.org/10.1016/j.carbpol.2018.12.065.
Q. Luo, J.R. Zhang, H.B. Li, D.T. Wu, F. Geng, H. Corke, X.L. Wei, and R.Y. Gan, Antioxidants (Basel). 9, 785 (2020). https://doi.org/10.3390/antiox9090785.
A. Lunkov, B. Shagdarova, M. Konovalova, Y. Zhuikova, N. Drozd, A. Il’ina, and V. Varlamov, Carbohydr. Polym. 234, 115916 (2020). https://doi.org/10.1016/j.carbpol.2020.115916.
Y. Rong, B. Cao, B. Liu, W. Li, Y. Chen, H. Chen, Y. Liu, and T. Liu, Int. Immunopharmacol. 64, 183 (2018). https://doi.org/10.1016/j.intimp.2018.08.024.
S. Li, W. Tang, P. Shi, M. Li, J. Sun, and J. Gong, Cryst. Growth Des. 20, 3173 (2020). https://doi.org/10.1021/acs.cgd.0c00044.
M. Zhang, X. Zhang, C.T. Ho, and Q. Huang, J. Agric. Food Chem. 67, 5374 (2019). https://doi.org/10.1021/acs.jafc.8b04837.
P. Chaikul, N. Khat-udomkiri, K. Iangthanarat, J. Manosroi, and A. Manosroi, Eur. J. Pharm. Sci. 131, 39 (2019). https://doi.org/10.1016/j.ejps.2019.02.008.
J. Li, S.Y. Kim, X. Chen, and H.J. Park, LWT Food Sci. Technol. 68, 667 (2016). https://doi.org/10.1016/j.lwt.2016.01.012.
N.A.A. Zahrani, R.M. El-Shishtawy, and A.M. Asiri, Eur. J. Med. Chem. 204, 112609 (2020). https://doi.org/10.1016/j.ejmech.2020.112609.
F.H.A. Fernandes, and H.R.N. Salgado, Crit. Rev. Anal. Chem. 46, 257 (2015). https://doi.org/10.1080/10408347.2015.1095064.
J. Yang, Z. Li, T. Guang, M. Hu, R. Cheng, R. Wang, C. Shi, J. Chen, P. Hou, K. Zhu, and X. Wang, Green Chem. 20, 5215 (2018). https://doi.org/10.1039/c8gc02584c.
A. Mahajan, A. Arya, and T.S. Chundawat, Synth. Commun. 49, 1926 (2019). https://doi.org/10.1080/00397911.2019.1610776.
O. Rocha-Rocha, M. Cortez-Valadez, A.R. Hernández-Martínez, R. Gámez-Corrales, R.A.B. Alvarez, R. Britto-Hurtado, Y. Delgado-Beleño, C.E. Martinez-Nuñez, A. Pérez-Rodríguez, H. Arizpe-Chávez, and M. Flores-Acosta, J. Electron. Mater. 46, 802 (2016). https://doi.org/10.1007/s11664-016-4942-2.
Y. Wang, D. O’Connor, Z. Shen, I.M.C. Lo, D.C.W. Tsang, S. Pehkonen, S. Pu, and D. Hou, J. Clean. Prod. 22, 540–549 (2019). https://doi.org/10.1016/j.jclepro.2019.04.128.
C.W. Han, M. Ma, H.H. Zhang, M. Li, and Q.J. Sun, Food Chem. 308, 125676 (2020). https://doi.org/10.1016/j.foodchem.2019.125676.
C.B.V. Buiten, J.D. Lambert, and R.J. Elias, Mol. Nutr. Food Res. 62, e1700879 (2018). https://doi.org/10.1002/mnfr.201700879.
P. Manivasagan, S.Y. Nam, and J. Oh, Crit. Rev. Microbiol. 42, 1007 (2016). https://doi.org/10.3109/1040841x.2015.1137860.
H.E. Emam, and M.M. El-Zawahry, Carbohydr. Polym. 166, 1 (2017). https://doi.org/10.1016/j.carbpol.2017.02.091.
W. Lu, Y. Sun, H. Dai, P. Ni, S. Jiang, Y. Wang, Z. Li, and Z. Li, Sens. Actuat. B Chem. 231, 860 (2016). https://doi.org/10.1016/j.snb.2016.03.058.
Acknowledgments
We thank the National Natural Science Foundation of China (No. 51873175), the Young Teachers Research Capacity Promotion Program of Northwest Normal University (NWNU-LKQN-16-19), Special Foundation projects for guiding technological innovation and development of Gansu Province (No. 2019ZX-05), and the University research and innovation team project of Gansu Province (No. 2018C-04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tian, W., Ding, X., Jiang, F. et al. Green Preparation of Cu Nanoparticles via Gallic Acid Applied to H2O2 Detection. J. Electron. Mater. 51, 1752–1758 (2022). https://doi.org/10.1007/s11664-021-09200-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-021-09200-3