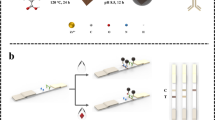
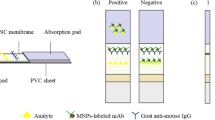
Abstract
A sensitive immunochromatographic assay (ICA) using time-resolved fluorescence microspheres (TRFMs) coupled with an indirect-labeling mode was developed for simultaneously determining 22 kinds of β-lactams in milk samples. The TRFMs labeled anti-receptor monoclonal antibodies (mAbs) conjugated to penicillin-binding proteins (PBPs) as ternary TRFMs-mAb-PBPs (TMP) nanoscaffolds provide excellent solubility, brightness, and stability. Thanks to the fact that they not only fully expose the binding sites of PBPs, thereby enhancing the biological affinity of PBPs towards the target, but also generated superb fluorescence signals, the versatile TMP manifested unique possibilities as efficient probes for ICA with remarkable enhancement in sensitivity in β-lactams screening. The results showed that the standard curves of the 22 varying β-lactams displayed linearity in their respective concentration ranges (R2 > 0.98), with the cutoff values of 1–100 ng/mL. The constructed TMP-ICA was successfully applied to the analysis of real milk, with consistent results compared with liquid chromatography-tandem mass spectrometry (LC-MS), providing an effective method for sensing β-lactams in food matrices.
Graphical Abstract







Similar content being viewed by others
References
Kolhe P, Roberts A, Gandhi S (2023) Fabrication of an ultrasensitive electrochemical immunosensor coupled with biofunctionalized zero-dimensional graphene quantum dots for rapid detection of cephalexin. Food Chem 398:133846. https://doi.org/10.1016/j.foodchem.2022.133846
Peris-Vicente J, Peris-Garcia E, Albiol-Chiva J, Durgbanshi A, Ochoa-Aranda E, Carda-Broch S, Bose D, Esteve-Romero J (2022) Liquid chromatography, a valuable tool in the determination of antibiotics in biological, food and environmental samples. Microchem J 177:107309. https://doi.org/10.1016/j.microc.2022.107309
Zhang X, Zhao F, Sun Y, Mi T, Wang L, Li Q, Li J, Ma W, Liu W, Zuo J et al (2020) Development of a highly sensitive lateral flow immunoassay based on receptor-antibody-amorphous carbon nanoparticles to detect 22 beta-lactams in milk. Sensors Actuators B Chem 321:128458. https://doi.org/10.1016/j.snb.2020.128458
Li Y, Liu L, Xu C, Kuang H, Sun L (2021) Integration of antibody-antigen and receptor-ligand reactions to establish a gold strip biosensor for detection of 33 beta-lactam antibiotics. Sci China Mater 64(8):2056–2066. https://doi.org/10.1007/s40843-020-1578-0
Li Y, Xu X, Liu L, Kuang H, Xu L, Xu C (2020) Rapid detection of 21 beta-lactams using an immunochromatographic assay based on the mutant BlaR-CTD protein from Bacillus Licheniformis. Analyst 145(9):3257–3265. https://doi.org/10.1039/d0an00421a
Di Rocco M, Moloney M, O’Beirne T, Earley S, Berendsen B, Furey A, Danaher M (2017) Development and validation of a quantitative confirmatory method for 30 β-lactam antibiotics in bovine muscle using liquid chromatography coupled to tandem mass spectrometry. J Chromatogr A 1500:121–135. https://doi.org/10.1016/j.chroma.2017.04.022
Melekhin AO, Tolmacheva VV, Goncharov NO, Apyari VV, Dmitrienko SG, Shubina EG, Grudev AI (2022) Multi-class, multi-residue determination of 132 veterinary drugs in milk by magnetic solid-phase extraction based on magnetic hypercrosslinked polystyrene prior to their determination by high-performance liquid chromatography-tandem mass spectrometry. Food Chem 387:132866. https://doi.org/10.1016/j.foodchem.2022.132866
Nunes MJ, Paz V, Cordas CM, Noronha JP, Branco LC (2022) LC-MS/MS methodology development and validation for the screening and quantification of five antibiotics in water. Anal Methods 14(9):935–948. https://doi.org/10.1039/d1ay01754c
Fridlund J, Woksepp H, Schon T (2016) A microbiological method for determining serum levels of broad spectrum beta-lactam antibiotics in critically ill patients. J Microbiol Methods 129:23–27. https://doi.org/10.1016/j.mimet.2016.07.020
Quintero-Campos P, Jose Juarez M, Morais S, Maquieira A (2020) Multiparametric highly sensitive chemiluminescence immunoassay for quantification of beta-lactam-specific immunoglobulin E. Anal Chem 92(21):14608–14615. https://doi.org/10.1021/acs.analchem.0c03020
Bai Y, Dou L, Wu W, Lu Z, Kou J, Shen J, Wen K, Wang Z (2021) Anti-metatype antibody screening, sandwich immunoassay development, and structural insights for beta-lactams based on penicillin binding protein. Molecules 26(18):5569. https://doi.org/10.3390/molecules26185569
Viter R, Savchuk M, Iatsunskyi I, Pietralik Z, Starodub N, Shpyrka N, Ramanaviciene A, Ramanavicius A (2018) Analytical, thermodynamical and kinetic characteristics of photoluminescence immunosensor for the determination of Ochratoxin A. Biosens Bioelectron 99:237–243. https://doi.org/10.1016/j.bios.2017.07.056
Myndrul V, Viter R, Savchuk M, Shpyrka N, Erts D, Jevdokimovs D, Silamiķelis V, Smyntyna V, Ramanavicius A, Iatsunskyi I (2018) Porous silicon based photoluminescence immunosensor for rapid and highly-sensitive detection of Ochratoxin A. Biosens Bioelectron 102:661–667. https://doi.org/10.1016/j.bios.2017.11.048
Myndrul V, Viter R, Savchuk M, Koval M, Starodub N, Silamiķelis V, Smyntyna V, Ramanavicius A, Iatsunskyi I (2017) Gold coated porous silicon nanocomposite as a substrate for photoluminescence-based immunosensor suitable for the determination of Aflatoxin B1. Talanta 175:297–304. https://doi.org/10.1016/j.talanta.2017.07.054
Li X, Pan Z, Li M, Jia X, Zhang S, Lin H, Ma L (2020) Europium chelate-labeled lateral flow assay for rapid and multiple detection of beta-lactam antibiotics by the penicillin-binding protein. Anal Methods 12(28):3645–3653. https://doi.org/10.1039/d0ay01140a
Lei X, Xu X, Liu L, Wang L, Kuang H, Xu C (2023) Gold-nanoparticle-based multiplex immuno-strip biosensor for simultaneous determination of 83 antibiotics. Nano Res 16(1):1259–1268. https://doi.org/10.1007/s12274-022-4762-z
Komova NN, Berlina A, Zherdev A, Dzantiev B (2020) Immune recognition of closed and open lactam rings and their influence on immunoassays of ampicillin antibiotics. Orient J Chem 36(1):21–25. https://doi.org/10.13005/ojc/360103
Chen H, Wu J, Zhou M, Zhou H, Li X, Chen X, Zou H, Guo Q, Xiong Y (2023) Ultrabright red-emitted aggregation-induced luminescence microspheres-based lateral flow immunoassay for furosemide detection in slimming products. Microchem J 190. https://doi.org/10.1016/j.microc.2023.108591
Liang M, Cai X, Gao Y, Yan H, Fu J, Tang X, Zhang Q, Li P (2022) A versatile nanozyme integrated colorimetric and photothermal lateral flow immunoassay for highly sensitive and reliable Aspergillus flavus detection. Biosens Bioelectron 213:114435. https://doi.org/10.1016/j.bios.2022.114435
Bai F, Bu T, Zhao S, He K, Zhang H, Li R, Li M, Wang Y, Wang L (2022) Golf-shaped Bi2Se3 microparticles based-immunochromatographic strip for ultrasensitive detection of Acetamiprid. J Hazard Mater 433:128810. https://doi.org/10.1016/j.jhazmat.2022.128810
Bu T, Bai F, Zhao S, Sun X, Jia P, He K, Wang Y, Li Q, Wang L (2022) Dual-modal immunochromatographic test for sensitive detection of zearalenone in food samples based on biosynthetic Staphylococcus aureus-mediated polymer dot nanocomposites. Anal Chem 94(14):5546–5554. https://doi.org/10.1021/acs.analchem.1c04721
Dang M, Li Z, Mao Y, Huang X, Song L, Li W, Ma R, Liu Y, Wang L, Yu X et al (2023) A highly sensitive lateral flow immunoassay based on a group-specific monoclonal antibody and amorphous carbon nanoparticles for detection of sulfonamides in milk. Microchim Acta 190(5):186. https://doi.org/10.1007/s00604-023-05766-3
Su C, Ding F, Wang W, Song Z, Ali Q, Ali M, Hong N, Wang G, Han H (2022) Time-resolved fluorescent microsphere lateral flow biosensors for rapid detection of Candidatus Liberibacter asiaticus. Plant Biotechnol J 20(7):1235–1237. https://doi.org/10.1111/pbi.13828
Mao Y, Fan Y, Yang R, Wang Y, Li Q, Dang M, Huang X, Song L, Zhang P, Song M et al (2023) A highly sensitive electrochemical sensor derived from peptide amphiphilic inspired self-assembled, ordered gold nanoparticles for determination of 22 β-lactams. Electrochim Acta 461:142669. https://doi.org/10.1016/j.electacta.2023.142669
Shen R, Guan T, Li Z, Hong Z, Dzantiev BB, Zherdev AV, Koidis A, Yao X, Lei H (2022) Identifying an emergent adulterant hydrochlorothiazide in food: a simple lateral flow strip with high sensitivity by time-resolved fluorescence. Food Control 143:109265. https://doi.org/10.1016/j.foodcont.2022.109265
Zhang X, Ding M, Mao Y, Huang X, Xie X, Song L, Qiao M, Zhang J, Wang T, Zhu H et al (2022) A comparative study of “turn-off” mode and “turn-on” mode lateral flow immunoassay for T-2 toxin detection. Sensors Actuators B Chem 359:131545. https://doi.org/10.1016/j.snb.2022.131545
Funding
This work was supported by the Key Scientific and Technological Project of Henan Provincial Education Department of China (222102310162), the Henan Postgraduate Joint Training Base Project (YJS2022JD16), and the Program for Innovative Research Team (in Science and Technology), University of Henan Province (No. 23IRTSTHN023).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 351 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, Y., Deng, X., Luo, C. et al. Time-resolved fluorescence microspheres-antibody-penicillin-binding protein assisted construction of immunochromatographic assay for sensitive detection of 22 β-lactams in milk. Microchim Acta 191, 50 (2024). https://doi.org/10.1007/s00604-023-06106-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-06106-1