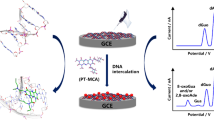
Abstract
T4 polynucleotide kinase (T4 PNK) helps with DNA recombination and repair. In this work, a phosphate pillar[5]arene@palladium nanoparticles@reduced graphene oxide nanocomposite (PP5@PdNPs@rGO)–based electrochemical biosensor was created to identify T4 PNK activities. The PP5 used to complex toluidine blue (TB) guest molecules is water-soluble. With T4 PNK and ATP, the substrate DNA, which included a 5ʹ-hydroxyl group, initially self-assembled over the gold electrode surface by chemical adsorption of the thiol units. Strong phosphate-Zr4+-phosphate chemistry allowed Zr4+ to act as a bridge between phosphorylated DNA and PP5@PdNPs@rGO. Through a supramolecular host–guest recognition connection, TB molecules were able to penetrate the PP5 cavity, where they produced a stronger electrochemical response. With a 5 × 10−7 U mL−1 detection limit, the electrochemical signal is linear in the 10−6 to 1 U mL−1 T4 PNK concentration range. It was also effective in measuring HeLa cell lysate–related PNK activities and screening PNK inhibitors. Nucleotide kinase–target drug development, clinical diagnostics, and screening for inhibitors all stand to benefit greatly from the suggested technology, which offers a unique sensing mechanism for kinase activity measurement.
Graphical Abstract








Similar content being viewed by others
Data availability
Data will be made available on request.
References
Wang LK, Lima CD, Shuman S (2002) Structure and mechanism of T4 polynucleotide kinase: an RNA repair enzyme. EMBO J 21(14):3873–3880. https://doi.org/10.1093/emboj/cdf397
Bernstein NK, Williams RS, Rakovszky ML, Cui D, Green R, Karimi-Busheri F, Mani RS, Galicia S, Koch CA, Cass CE, Durocher D, Weinfeld M, Glover JNM (2005) The molecular architecture of the mammalian DNA repair enzyme, polynucleotide kinase. Mol Cell 17(5):657–670. https://doi.org/10.1016/j.molcel.2005.02.012
Tahbaz N, Subedi S, Weinfeld M (2012) Role of polynucleotide kinase/phosphatase in mitochondrial DNA repair. Nucleic Acids Res 40(8):3484–3495. https://doi.org/10.1093/nar/gkr1245
Sallmyr A, Rashid I, Bhandari SK, Naila T, Tomkinson AE (2020) Human DNA ligases in replication and repair. DNA Repair 93:102908. https://doi.org/10.1016/j.dnarep.2020.102908
Dumitrache LC, McKinnon PJ (2017) Polynucleotide kinase-phosphatase (PNKP) mutations and neurologic disease. Mech Ageing Dev 161:121–129. https://doi.org/10.1016/j.mad.2016.04.009
Chatterjee A, Saha S, Chakraborty A, Silva-Fernandes A, Mandal SM, Neves-Carvalho A, Liu Y, Pandita RK, Hegde ML, Hegde PM, Boldogh I, Ashizawa T, Koeppen AH, Pandita TK, Maciel P, Sarkar PS, Hazra TK (2015) The role of the mammalian DNA end-processing enzyme polynucleotide kinase 3’-phosphatase in spinocerebellar Ataxia type 3 pathogenesis. PLOS Genet 11(1):e1004749. https://doi.org/10.1371/journal.pgen.1004749
Yannone SM, Roy S, Chan DW, Murphy MB, Huang S, Campisi J, Chen DJ (2001) Werner syndrome protein is regulated and phosphorylated by DNA-dependent protein kinase. J Biol Chem 276(41):38242–38248. https://doi.org/10.1074/jbc.m101913200
Shen J, Gilmore EC, Marshall CA, Haddadin M, Reynolds JJ, Eyaid W, Bodell A, Barry B, Gleason D, Allen K, Ganesh VS, Chang BS, Grix A, Hill RS, Topcu M, Caldecott KW, Barkovich AJ, Walsh CA (2010) Mutations in PNKP cause microcephaly, seizures and defects in DNA repair. Nat Genet 42:245–249. https://doi.org/10.1038/ng.526
Kalasova I, Hailstone R, Bublitz J, Bogantes J, Hofmann W, Leal A, Hanzlikova H, Caldecott KW (2020) Pathological mutations in PNKP trigger defects in DNA single-strand break repair but not DNA double-strand break repair. Nucleic Acids Res 48(12):6672–6684. https://doi.org/10.1093/nar/gkaa489
Freschauf GK, Karimi-Busheri F, Ulaczyk-Lesanko A, Mereniuk TR, Ahrens A, Koshy JM, Rasouli-Nia A, Pasarj P, Holmes CF, Rininsland F, Hall DG, Weinfeld M (2009) Identification of a small molecule inhibitor of the human DNA repair enzyme polynucleotide kinase/phosphatase. Cancer Res 69(19):7739–7746. https://doi.org/10.1158/0008-5472.can-09-1805
Mereniuk TR, Maranchuk RA, Schindler A, Penner-Chea J, Freschauf GK, Hegazy S, Lai R, Foley E, Weinfeld M (2012) Genetic screening for synthetic lethal partners of polynucleotide kinase/phosphatase: potential for targeting SHP-1-Depleted cancers. Cancer Res 72(22):5934–5944. https://doi.org/10.1158/0008-5472.can-12-0939
Mereniuk TR, El Gendy MAM, Mendes-Pereira AM, Lord CJ, Ghosh S, Foley E, Ashworth A, Weinfeld M (2013) Synthetic lethal targeting of PTEN-deficient cancer cells using selective disruption of polynucleotide kinase/phosphatase. Mol Cancer Ther 12(10):2135–2144. https://doi.org/10.1158/1535-7163.mct-12-1093
Rasouli-Nia A, Karimi-Busheri F, Weinfeld M (2004) Stable down-regulation of human polynucleotide kinase enhances spontaneous mutation frequency and sensitizes cells to genotoxic agents. Proc Nat Acad Sci USA 101(18):6905–6910. https://doi.org/10.1073/pnas.0400099101
Karimi-Busheri F, Rasouli-Nia A, Allalunis-Turner J, Weinfeld M (2007) Human polynucleotide kinase participates in repair of DNA double-strand breaks by nonhomologous end joining but not homologous recombination. Cancer Res 67(14):6619–6625. https://doi.org/10.1158/0008-5472.can-07-0480
Phillips DH, Arlt VM (2007) The 32P-postlabeling assay for DNA adducts. Nat Protoc 2:2772–2781. https://doi.org/10.1038/nprot.2007.394
Wang LK, Shuman S (2001) Domain structure and mutational analysis of T4 polynucleotide kinase. J Biol Chem 276(29):26868–26874. https://doi.org/10.1074/jbc.m103663200
Chappell C, Hanakahi LA, Karimi-Busheri F, Weinfeld M, West SC (2002) Involvement of human polynucleotide kinase in double-strand break repair by non-homologous end joining. EMBO J 21:2827–2832. https://doi.org/10.1093/emboj/21.11.2827
Whitehouse CJ, Taylor RM, Thistlethwaite A, Zhang H, Karimi-Busheri F, Lasko DD, Weinfeld M, Caldecott KW (2001) XRCC1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair. Cell 104(1):107–117. https://doi.org/10.1016/s0092-8674(01)00195-7
Liu HS, Ma CB, Wang J, Chen HC, Wang KM (2017) Label-free colorimetric assay for T4 polynucleotide kinase/phosphatase activity and its inhibitors based on G-quadruplex/hemin DNAzyme. Anal Biochem 517:18–21. https://doi.org/10.1016/j.ab.2016.10.022
Lin L, Shi DM, Li QF, Wang GF, Zhang XJ (2016) Detection of T4 polynucleotide kinase based on a MnO2 nanosheet-3,3’,5,5’-tetramethylbenzidine (TMB) colorimetric system. Anal Methods 8:4119–4126. https://doi.org/10.1039/c6ay00269b
Zhu J, Chen LT (2022) Highly efficient incorporation of dATP in terminal transferase polymerization forming the ploy (A)n-DITO-1 fluorescent probe sensing terminal transferase and T4 polynucleotide kinase activity. Anal Chim Acta 1221:340080. https://doi.org/10.1016/j.aca.2022.340080
Zhang YQ, Cai QQ, Yan XS, Jie GF (2023) Versatile fluorescence detection of T4 PNK and mRNA based on unique DNA nanomachine amplification. Anal Chim Acta 1251:341003. https://doi.org/10.1016/j.aca.2023.341003
Xie ZW, Wang XY, Chen SY, Zhao ZX, Zhao SH, Zhang WX, Luo LJ, Yi G (2022) Construction of a simple, localized and homogeneous fluorescence detection platform for T4 PNK activity based on tetrahedral DNA nanostructure-mediated primer exchange reaction. Microchem J 183:107989. https://doi.org/10.1016/j.microc.2022.107989
Qin YQ, Ke WK, Zhou YN, Zhu DD, Li YJ, Hu YG (2023) TtAgo sensor for the sensitive and rapid detection of T4 polynucleotide kinase activity. Sens Actuators B Chem 386:133753. https://doi.org/10.1016/j.snb.2023.133753
Chai YY, Cheng X, Xu GH, Wei FD, Bao J, Mei J, Ren DD, Hu Q, Cen Y (2020) A nanoplatform based on metal–organic frameworks and coupled exonuclease reaction for the fluorimetric determination of T4 polynucleotide kinase activity and inhibition. Microchim Acta 187:243. https://doi.org/10.1007/s00604-020-4194-y
Cao Y, Zhou Y, Lin YH, Zhu JJ (2021) Hierarchical metal−organic framework-confined CsPbBr 3 quantum dots and aminated carbon dots: a new self-sustaining suprastructure for electrochemiluminescence bioanalysis. Anal Chem 93:1818–1825. https://doi.org/10.1021/acs.analchem.0c04717
Zhang GY, Chai HN, Tian MW, Zhu SF, Qu LJ, Zhang XJ (2020) Zirconium−metalloporphyrin frameworks−luminol competitive electrochemiluminescence for ratiometric detection of polynucleotide kinase activity. Anal Chem 92:7354–7362. https://doi.org/10.1021/acs.analchem.0c01262
Yang JH, He GH, Wu WY, Deng WF, Tan YM, Xie QJ (2022) Sensitive photoelectrochemical determination of T4 polynucleotide kinase using AuNPs/SnS2/ZnIn2S4 photoactive material and enzymatic reaction-induced DNA structure switch strategy. Talanta 249:123660. https://doi.org/10.1016/j.talanta.2022.123660
Li PP, Cao Y, Mao CJ, Jin BK, Zhu JJ (2019) TiO2/g-C3N4/CdS nanocomposite-based photoelectrochemical biosensor for ultrasensitive evaluation of T4 polynucleotide kinase activity. Anal Chem 91:1563–1570. https://doi.org/10.1021/acs.analchem.8b04823
Tao JP, Liu ZQ, Zhu ZY, Zhang YL, Wang HB, Pang PF, Yang C, Yang WR (2022) Electrochemical detection of T4 polynucleotide kinase activity based on magnetic Fe3O4@TiO2 nanoparticles triggered by a rolling circle amplification strategy. Talanta 241:123272. https://doi.org/10.1016/j.talanta.2022.123272
Yan ZY, Shen XY, Zhou BL, Pan RY, Zhang B, Zhao CZ, Ren LH, Ming JJ (2021) Precise analysis of T4 polynucleotide kinase and inhibition by coupling personal glucose meter with split DNAzyme and ligation-triggered DNA walker. Sens Actuators B Chem 326:128831. https://doi.org/10.1016/j.snb.2020.128831
Mao JX, Chen X, Xu HH, Xu XQ (2020) DNAzyme-driven DNA walker biosensor for amplified electrochemical detection of T4 polynucleotide kinase activity and inhibition. J Electroanal Chem 874:114470. https://doi.org/10.1016/j.jelechem.2020.114470
Yan ZY, Deng PY, Liu Y (2019) Recent advances in protein kinase activity analysis based on nanomaterials. Int J Mol Sci 20(6):1440. https://doi.org/10.3390/ijms20061440
Song Z, Li Y, Teng H, Ding CF, Xu GY, Luo XL (2020) Designed zwitterionic peptide combined with sacrificial Fe-MOF for low fouling and highly sensitive electrochemical detection of T4 polynucleotide kinase. Sens Actuators B Chem 305:127329. https://doi.org/10.1016/j.snb.2019.127329
Li JL, Ma JH, Zhang YC, Zhang ZL, He GW (2019) A fluorometric method for determination of the activity of T4 polynucleotide kinase by using a DNA-templated silver nanocluster probe. Microchim Acta 186:48. https://doi.org/10.1007/s00604-018-3157-z
Zhang YL, Fang X, Zhu ZY, Lai YQ, Xu CL, Pang PF, Wang HB, Yang C, Barrow CJ, Yang WR (2018) A sensitive electrochemical assay for T4 polynucleotide kinase activity based on titanium dioxide nanotubes and a rolling circle amplification strategy. RSC Adv 8(67):38436–38444. https://doi.org/10.1039/c8ra07745b
Song WL, Yin WS, Zhang ZH, He P, Yang XY, Zhang XR (2019) A DNA functionalized porphyrinic metal-organic framework as a peroxidase mimicking catalyst for amperometric determination of the activity of T4 polynucleotide kinase. Microchim Acta 186:149. https://doi.org/10.1007/s00604-019-3269-0
Lin MH, Wan H, Zhang J, Wang Q, Hu XY, Xia F (2020) Electrochemical DNA sensors based on MoS2-AuNPs for polynucleotide kinase activity and inhibition assay. ACS Appl Mater Interfaces 12:45814–45821. https://doi.org/10.1021/acsami.0c13385
Zhou YL, Yin HS, Zhao WW, Ai SY (2020) Electrochemical, electrochemiluminescent and photoelectrochemical bioanalysis of epigenetic modifiers: a comprehensive review. Coordin Chem Rev 424:213519. https://doi.org/10.1016/j.ccr.2020.213519
Ma XH, Hao YQ, Dong XX, Xia N (2023) Biosensors with metal ion–phosphate chelation interaction for molecular recognition. Molecules 28(11):4394. https://doi.org/10.3390/molecules28114394
Liao Y, Zhang YQ, Su AW, Zhang YL, Wang HB, Yang WR, Pang PF (2023) Zr4+-mediated DNAzyme-driven DNA walker amplification strategy for electrochemical assay of protein kinase a activity and inhibition. Talanta 260:124612. https://doi.org/10.1016/j.talanta.2023.124612
Hu Q, Kong JM, Han DX, Bao Y, Zhang XJ, Zhang YW, Niu L (2020) Ultrasensitive peptide-based electrochemical detection of protein kinase activity amplified by RAFT polymerization. Talanta 206:120173. https://doi.org/10.1016/j.talanta.2019.120173
Ohtani S, Kato K, Fa S, Ogoshi T (2022) Host–guest chemistry based on solid-state pillar[n]arenes. Coord Chem Rev 462:214503. https://doi.org/10.1016/j.ccr.2022.214503
Sathiyajith C, Shaikh RR, Han Q, Zhang Y, Meguellati K, Yang YW (2017) Biological and related applications of pillar[n]arenes. Chem Commun 53:677–696. https://doi.org/10.1039/c6cc08967d
Yang K, Pei YX, Wen J, Pei ZC (2016) Recent advances in pillar[n]arenes: synthesis and applications based on host-guest interactions. Chem Commun 52:9316–9326. https://doi.org/10.1039/c6cc03641d
Shamagsumova RV, Shurpik DN, Kuzin YI, Stoikov II, Rogov AM, Evtugyn GA (2022) Pillar[6]arene: electrochemistry and application in electrochemical (bio)sensors. J Electroanal Chem 913:116281. https://doi.org/10.1016/j.jelechem.2022.116281
Cao S, Zhou L, Liu C, Zhang HC, Zhao YX, Zhao YL (2021) Pillararene-based self-assemblies for electrochemical biosensors. Biosens Bioelectron 181:113164. https://doi.org/10.1016/j.bios.2021.113164
Evtyugin GA, Shurpik DN, Stoikov II (2020) Electrochemical sensors and biosensors on the pillar[5]arene platform. Russ Chem Bull Int Ed 69:859–874. https://doi.org/10.1007/s11172-020-2843-2
Wang J, Zhou L, Bei JL, Zhao QY, Li X, He JQ, Cai Y, Chen TT, Du YK, Yao Y (2022) An enhanced photo-electrochemical sensor constructed from pillar [5]arene functionalized Au NPs for ultrasensitive detection of caffeic acid. Talanta 243:123322. https://doi.org/10.1016/j.talanta.2022.123322
Wang J, Bei JL, Guo X, Ding Y, Chen TT, Lu B, Wang Y, Du YK, Yao Y (2022) Ultrasensitive photoelectrochemical immunosensor for carcinoembryonic antigen detection based on pillar[5]arene-functionalized Au nanoparticles and hollow PANI hybrid BiOBr heterojunction. Biosens Bioelectron 208:114220. https://doi.org/10.1016/j.bios.2022.114220
Liang H, Zhao YT, Ye HZ, Li CP (2019) Ultrasensitive and ultrawide range electrochemical determination of bisphenol A based on PtPd bimetallic nanoparticles and cationic pillar[5]arene decorated graphene. J Electroanal Chem 855:113487. https://doi.org/10.1016/j.jelechem.2019.113487
Tan XP, He SH, Liu X, Zhao GF, Huang T, Yang L (2019) Ultrasensitive electrochemical sensing of dopamine by using dihydroxylatopillar[5]arene-modified gold nanoparticles and anionic pillar[5]arene-functionalized graphitic carbon nitride. Microchim Acta 186:703. https://doi.org/10.1007/s00604-019-3869-8
Hu X, Liu X, Zhang W, Qin S, Yao C, Li Y, Cao D, Peng L, Wang L (2016) Controllable construction of biocompatible supramolecular micelles and vesicles by water-soluble phosphate pillar[5,6]arenes for selective anti-cancer drug delivery. Chem Mater 28:3778–3788. https://doi.org/10.1021/acs.chemmater.6b00691
Luo D, Liu ZQ, Su AW, Zhang YL, Wang HB, Yang LJ, Yang WR, Pang PF (2024) An electrochemical biosensor for detection of T4 polynucleotide kinase activity based on host-guest recognition between phosphate pillar[5]arene and methylene blue. Talanta 266:124956. https://doi.org/10.1016/j.talanta.2023.124956
Qian XC, Tan S, Li Z, Qu Q, Li L, Yang L (2020) A robust host-guest interaction controlled probe immobilization strategy for the ultrasensitive detection of HBV DNA using hollow HP5-Au/CoS nanobox as biosensing platform. Biosens Bioelectron 153:112051. https://doi.org/10.1016/j.bios.2020.112051
Yang L, Zhao H, Li YC, Ran X, Deng GG, Zhang YQ, Ye HZ, Zhao GF, Li CP (2016) Indicator displacement assay for cholesterol electrochemical sensing using a calix[6]arene functionalized graphene-modified electrode. Analyst 141:270–278. https://doi.org/10.1039/c5an01843a
Zhao H, Yan Y, Chen MJ, Hu TT, Wu KF, Liu HS, Ma CB (2019) Exonuclease III-assisted signal amplification strategy for sensitive fluorescence detection of polynucleotide kinase based on poly(thymine)-templated copper nanoparticles. Analyst 144:6689–6697. https://doi.org/10.1039/c9an01659g
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 21665027, 21565031), Applied Basic Research Project of Yunnan Provincial Science and Technology Department (No. 202001AT070012), Graduate Scientific Research Foundation of Yunnan Minzu University (Nos. 2023SKY028, 2023SKY027), and YMU-DEAKIN International Associated Laboratory on Functional Materials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Y., Yi, J., Su, A. et al. An electrochemical biosensor for T4 polynucleotide kinase activity identification according to host–guest recognition among phosphate pillar[5]arene@palladium nanoparticles@reduced graphene oxide nanocomposite and toluidine blue. Microchim Acta 190, 394 (2023). https://doi.org/10.1007/s00604-023-05983-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05983-w