Abstract
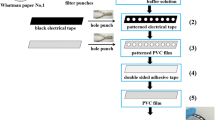
A cloth-based analytical device combined with electrochemiluminescence detection (CAD–ECL) was described for rapid determination of histamine (HA). The CAD device was produced by screen-printing a conductive carbon ink onto a patterned hydrophobic electrochemical microfluidic chamber to fabricate the three-carbon electrode system on a single hydrophilic cloth. The introduction of carbon nanodots linked to chitosan on the working carbon electrode surface enhanced the catalytic performance and overcame the resistance of the cotton fiber material. On this basis, the enhancement of the electrochemiluminescence (ECL) signal of the tris(2,2′-bipyridyl) ruthenium(II) complex, caused by HA, was observed in a phosphate buffer solution at pH 7.6. The proposed CAD–ECL sensor was successfully applied to the quantification of HA in fish and fishery samples with good linearity between ECL intensity and the logarithm of HA concentration in the range 1.0 to 1000.0 µg L−1 with a low detection limit of 0.82 µg L−1.
Graphical abstract








Similar content being viewed by others
Data Availability
Data of this reasearch is included in the manuscript and supplementary material, any other data can be provided upon request.
References
Becker K, Southwick K, Reardon J, Berg R, MacCormack JN (2001) Histamine poisoning associated with eating tuna burgers. J Am Med Assoc 285:2977–2978
EC Commission Regulation No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs, Official Journal of European Union, 2005
Feng C, Teuber S, Gershwin M (2016) Histamine (Scombroid) Fish Poisoning: a Comprehensive Review. Clin Rev Allergy Immunol 50:64–69
Fen NY, Sali AT, Ahmad R, Tze LM, Abdullah WNW (2011) Origin of proteolytic enzymes involved in production of Malaysian fish sauce, Budu. Thai J Agric Sci 44:542–547
FDA fish and fishery products hazards and controls guidance, Fourth Edition, Chapter 7, 113, April 2011
EU Commission Regulation No 1019/2013 of 23 October 2013, amending Annex I to Regulation (EC) No 2073/2005 as regards histamine in fishery products, Offic J E U 2013. http://faolex.fao.org/docs/pdf/eur127944.pdf. Accessed 30 Sept 2022
Brillantes S, Samosorn W (2001) Determination of histamine in fish sauce from Thailand using a solid phase extraction and high-performance liquid chromatography. Fish Sci 67:1163–1168
Rogers P, Staruszkiewicz W (2000) Histamine test kit comparison. J Aquat Food Prod Technol 9:5–17
Adamou R, Coly A, Douabalé SE, Tine A, Zamel ML, Seye MD (2005) Fluorimetric determination of histamine in halieutic products by using fluorescamine in micellar media. J Fluoresc 15:679–688
Pais GL, Meloni D, Mudadu AG, Crobu L, Pulina A, Chessa G (2022) Colorimetric analysis and determination of histamine in samples of yellowfin Tuna (Thunnus albacares) marketed in sardinia (Italy) by a combination of rapid screening methods and LC-MS/MS. Foods 11(5):639. https://doi.org/10.3390/foods11050639
Kvasnička F, Kavková S, Honzlová A (2019) Electrophoretic determination of histamine. J Chromatogr A 1588:180–184
Guan W, Zhang C, Liu F, Liu M (2015) Chemiluminescence detection for microfluidic cloth-based analytical devices (μCADs). Biosens Bioelectron 72:114–120
Li H, Wang D, Liu C, Liu R, Zhang C (2017) Facile and sensitive chemiluminescence detection of H2O2 and glucose by a gravity/capillary flow and cloth-based low-cost platform. RSC Adv 68:43245–43254
Guan W, Liu M, Zhang C (2016) Electrochemiluminescence detection in microfluidic cloth-based analytical devices. Biosens Bioelectron 75:247–253
Praoboon N, Senabut J, Thanomwat M, Tangkuaram T, Pookmanee P, Phaisansuthichol S, Sangsrichan S, Kuimalee S, Satienperakul S (2022) A cloth-based electrochemiluminescence sensor for determination of salbutamol residues in pork samples. Food Chemistry 386:132786. https://doi.org/10.1016/j.foodchem.2022.132786
Rasal AS, Yadav S, Yadav A, Kashale AA, Manjunatha ST, Altaee A, Chang J-Y (2021) Carbon quantum dots for energy applications: a review. ACS Appl Nano Mater 4:6515–6541
Broomhead JA, Young CG, Hood P (2007) Tris(2,2′-bipyridine) ruthenium(II) dichloride hexahydrate inorganic syntheses. Wiley, New York
Xu Z-Q, Lan J-Y, Jin J-C, Dong P, Jiang F-L, Liu Y (2015) Highly photoluminescent nitrogen-doped carbon nanodots and their protective effects against oxidative stress on cells. ACS Appl Mater Interfaces 7(51):28346–28352
Bandodkar AJ, Wang J (2014) Non-invasive wearable electrochemical sensors: a review. Trends Biotechnol 32:363–371
Singh A, Kaur A, Patra AK, Mahajan R (2018) A sustainable and green process for scouring of cotton fabrics using xylano-pectinolytic synergism: switching from noxious chemicals to eco-friendly catalysts. Biotech 8(4):184. https://doi.org/10.1007/s13205-018-1193-3
Nilghaz A, Wicaksono DHB, Gustiono D, Majid FAA, Supriyanto E, Kadira MRA (2012) Flexible microfluidic cloth-based analytical devices using a low-cost wax patterning technique. Lab Chip 12:209–218
Fan YZ, Zhang Y, Li N, Liu SG, Liu T, Li NB, Luo HQ (2017) A facile synthesis of water-soluble carbon dots as a label-free fluorescent probe for rapid, selective and sensitive detection of picric acid. Sensors Actuators B 240:949–955
Rigodanza F, Burian M, Arcudi F, Đorđević L, Amenitsch H, Prato M (2021) Snapshots into carbon dots formation through a combined soectroscopic approach. Nat Commun 12(1):2640. https://doi.org/10.1038/s41467-021-22902-w
Vasimalai N, Vilas-Boas V, Gallo J, Cerqueira MF, Menéndez-Miranda M, Costa-Fernández JM, Diéguez L, Espiña B, Fernández-Argüelles MT (2018) Green synthesis of fluorescent carbon dots from spices for in vitro imaging and tumour cell growth inhibition. Beilstein J Nanotechnol 9:530–544
Liu Y, Xiao N, Gong N, Wang H, Shi X, Gu W, Ye L (2014) One-step microwave-assisted polyol synthesis of green luminescent carbon dots as optical nanoprobes. Carbon 68:258–264
Sheng M, Gao Y, Sun J, Gao F (2014) Carbon nanodots–chitosan composite film: a platform for protein immobilization, direct electrochemistry and bioelectrocatalysis. Biosens Bioelectron 58:351–358
Begum H, Ahmed MS, Jeon S (2017) New approach for porous chitosan–graphene matrix preparation through enhanced amidation for synergic detection of dopamine and uric acid. ACS Omega 2(6):3043–3054
Chen Z, Zu Y (2008) Electrogenerated Chemiluminescence of the Tris(2,2′-bipyridine)ruthenium(II)/Tri-n-propylamine (TPrA) system: crucial role of the long lifetime of TPrA•+ cation radicals suggested by electrode surface effects. J Phys Chem C 112(42):16663–16667
Noffsinger JB, Danielson ND (1987) Generation of chemiluminescence upon reaction of aliphatic amines with tris(2,2’-bipyridine)ruthenium(III). Anal Chem 59(6):865–868
Heckenlaible N, Snyder S, Herchenbach P, Kava A, Henry CS, Gross EM (2022) Comparison of mobile phone and CCD cameras for electrochemiluminescent detection of biogenic amines. Sensors 22(18):7008. https://doi.org/10.3390/s22187008
Miller JC, Miller JN (2010) Statistics for analytical chemistry, 6th edn. Ashford Colour Press Ltd, Gosport, UK
Munir AM, Mackeen MMM, Heng LY, Bradi KH (2021) Study of histamine detection using liquid chromatography and gas chromatography. ASM Sci J 16:(1–9). https://doi.org/10.32802/asmscj.2021.809
Almeida C, Fernandes JO, Cunha SC (2012) A novel dispersive liquid–liquid microextraction (DLLME) gas chromatography-mass spectrometry (GC–MS) method for the determination of eighteen biogenic amines in beer. Food Control 25(1):380–388. https://doi.org/10.1016/j.foodcont.2011.10.052
Zhang X, Liu Q, Wang Z-W, Xu H, An F-P, Huang Q, Song H-B, Wang Y-W (2020) D-penicillamine modified copper nanoparticles for fluorometric determination of histamine based on aggregation-induced emission. Microchim Acta 187(6):329. https://doi.org/10.1007/s00604-020-04271-1
Pérez S, Bartrolí J, Fàbregas E (2013) Amperometric biosensor for the determination of histamine in fish samples. Food Chem 141(4):4066–4072. https://doi.org/10.1016/j.foodchem.2013.06.125
Xu L, Zhou J, Eremin S, Dias ACP, Zhang X (2020) Development of ELISA and chemiluminescence enzyme immunoassay for quantification of histamine in drug products and food samples. Anal Bioanal Chem 412(19):4739–4747. https://doi.org/10.1007/s00216-020-02730-5
Gao X, Gu X, Min Q, Wei Y, Tian C, Zhuang X, Luan F (2022) Encapsulating Ru(bpy)32+ in an infinite coordination polymer network: towards a solid-state electrochemiluminescence sensing platform for histamine to evaluate fish product quality. Food Chem 368:130852. https://doi.org/10.1016/j.foodchem.2021.130852
Praoboon N, Siriket S, Taokaenchan N, Tangkuaram T, Pookmanee P, Phaisansuthichol S, Kuimalee S, Satienperakul S (2022) Paper-based electrochemiluminescence device for the rapid estimation of trimethylamine in fish via the quenching effect of thioglycolic acid-capped cadmium selenide quantum dots. Food Chem 366:130590. https://doi.org/10.1016/j.foodchem.2021.130590
Tran QH, Nguyen T, Pham K (2020) Development of the high sensitivity and selectivity method for the determination of histamine in fish and fish sauce from Vietnam by UPLC-MS/MS. Int J Anal Chem, Article 2187646. https://doi.org/10.1155/2020/2187646
Ali A, Waheed K, Hadaiyt A, Begum I, Hayat S (2016) Determination of histamine levels by LC-MS/MS in various fish species available in the local markets of Punjab, Pakistan. Int J Fish Aquatic Stud 4(6):128–132
Acknowledgements
The authors are grateful to the Science and Technology Service Centre, Faculty of Science, Maejo University, for providing valuable assistance with the nanostructure investigations.
Funding
J. Senabut gratefully acknowledges financial support from the Rajamangala University and Technology Lanna Funding (RMUTL) for this work. Additionally, S. Satienperakul would like to thank the National Research Council of Thailand (NRCT) for their very kind support.
Author information
Authors and Affiliations
Contributions
All authors contributed to conceptualization of this manuscript. Jirapatpong Senabut methodology and design, formal analysis and investigation, and writing—original draft. Nisachon Praoboon: formal analysis and investigation. Tanin Tangkuaram: data curation and visualization. Pusit Pookmanee and Supaporn Sangsrichan: methodology and supervision. Surasak Kuimalee: visualization. Sakchai Satienperakul: methodology and design, formal analysis and investigation, validation, funding acquisition, resources, writing—review and editing, and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Senabut, J., Praoboon, N., Tangkuaram, T. et al. Development of cloth-based microfluidic devices for rapid determination of histamine in fish and fishery products. Microchim Acta 190, 213 (2023). https://doi.org/10.1007/s00604-023-05792-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05792-1