Abstract
A biosensor for rapid and simultaneous visual identification of high-risk human papillomavirus (HPV) genotypes 16 and 18 in clinical samples based on polymerase chain reaction (PCR) integrated lateral flow strip platform was developed. Using an one-step protocol to extract nucleic acid rapidly and the functionalized primer sets specific to HPV-16 and 18 were designed for the simultaneous amplification. In the presence of target HPV genotypes, the corresponding functionalized primer sets will participate in the PCR process and produce numerous duplex functionalized dsDNA amplicons. With the bridge effect of duplex functionalized dsDNA amplicons between gold nanoparticles-fluorescein isothiocyanate antibody conjugates (AuNP-FITC antibody conjugates) and other two antibodies on corresponding test line (T1 or T2), visualized color signals on test lines could be obtained directly visible with a naked eye. Combining the high amplification efficiency of PCR and the visualized sensing of LFS, as low as 700 copies of HPV-16 and 18 DNA were detected simultaneously within 75 min, which can promote application in the resource limited settings.
Graphical abstract
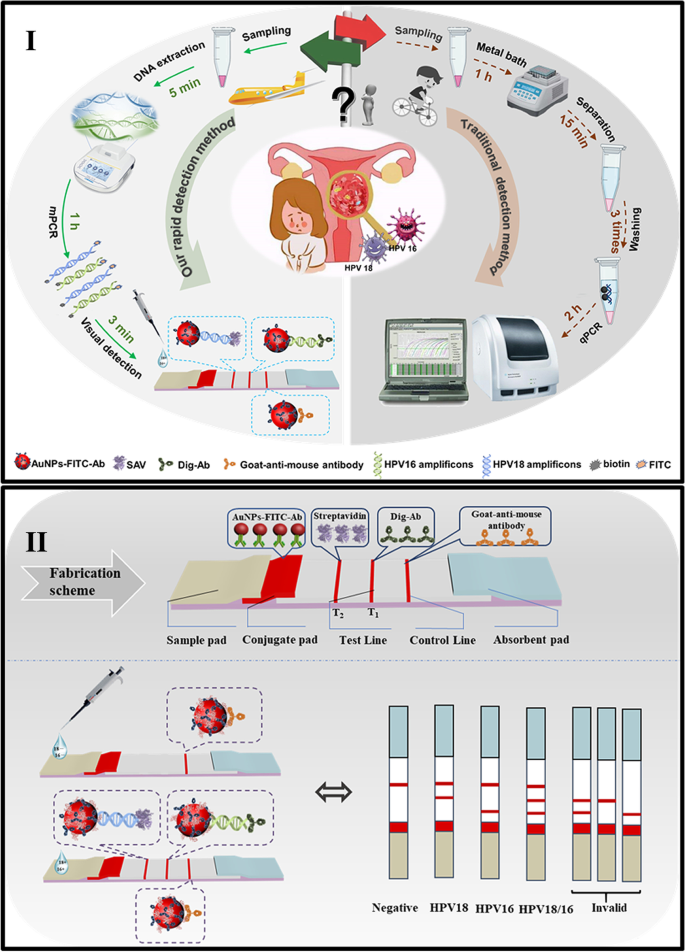
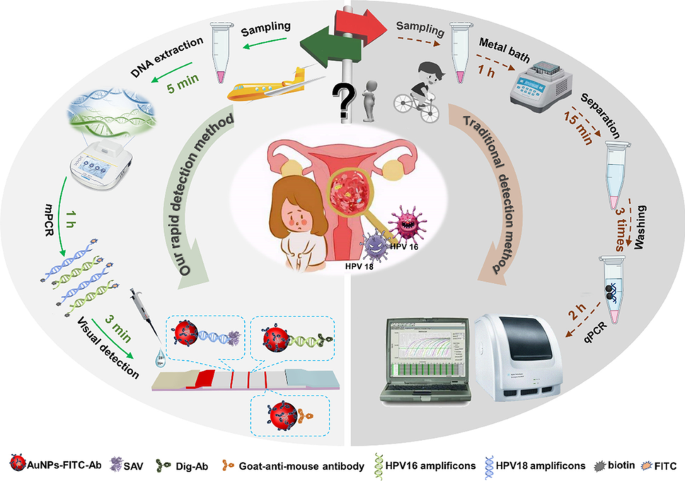
High-risk genotypes of HPV-16 and HPV-18 were easily and simultaneously screened with the amplification-assisted molecular lateral flow strip by on-site observation in the resource-limited settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early diagnosis and timely prevention of diseases, especially for cancer, are urgent issues in clinical monitoring. It is estimated that approximately 600,000 women are diagnosed with, and more than 300,000 women die from, cervical cancer worldwide annually [1]. Extensive research shows that HPV infection is the principal cause of cervical cancer, and over 99% of invasive cervical cancers are associated with high-risk HPV genotypes [2,3,4,5]. Recent evidences have strongly indicated that dentification of HPV genotypes is more efficient to screen and prevent invasive cervical cancer [6, 7]. It has been reported that 12 kinds of high-risk genotypes including HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 types have been classified as the group I carcinogens by the International Agency for Research on Cancer (IARC) [8, 9]. Among these HPV genotypes, HPV-16 is the highest risk one in cervical cancer cases compared to other high-risk HPV genotypes, which is followed by HPV-18 [3, 9]. Take all these into consideration, development of a rapid, reliable, and convenient method for routine screening or identification of HPV-16 and HPV-18 is of great significance for keeping health and early diagnosis.
Up to now, more than 150 methods have been reported for discriminating the HPV genotypes, but most of them have not come to the stage of clinical applications [7, 10, 11]. Among the current clinical adopted methods for HPV detection, the morphological and immunological methods were not effective enough with poor sensitivity and specificity and could also not be used for the identification of specific HPV genotypes [12]. Current gold standard methods for HPV genotyping were hybrid capture 2 (HC2) and polymerase chain reaction (PCR) [10]. Although HC2 was widely used for the detection of 13 kinds of HPV genotypes with high sensitivity, its specificity was greatly influenced by the cross-reaction with other types [13, 14]. PCR-based protocols were sensitive enough to achieve single and multiple HPV diagnosis [15]. Honestly, the post-treatment of PCR amplicons severely hampers its practical application in clinical diagnosis especially in the resource-limited setting in the developing countries, where the simplicity and the cost of methods are of greater importance. The agarose gel electrophoresis and probe hybridization protocols can only be adopted in the R&D process. Alternatively, the microarray and fluorescent measurement module have made PCR or real-time PCR extensively utilized in the professional labs. Due to the strict requirements about the location and operators and high cost of the hardware, these methods still cannot be widely applied in resource-limited settings [16,17,18,19]. Adoption of an easy-operational and low-cost strategy is of great significance to further widen the screening application in the field of in vitro diagnosis (IVD).
In clinical and point-of-care test (POCT), the colloidal lateral flow strip (LFS), especially the early pregnancy diagnosis LFS, can be defined as the first generation and the most successful commercialized products all over the world. And these LFS-based protocols and kits have been extensively applied in environmental monitoring, food safety, and also early diagnosis of various diseases due to its simplicity, efficiency and low cost [20,21,22,23,24,25]. Meanwhile, besides the gold nanoparticles, some functional nanomaterials including quantum dots (QDs) [26], multiwalled carbon nanotubes (MWCNTs) [27, 28], fluorescent microsphere [29], and magnetic beads [30] have been adopted as the signal indicators or signal enhancers in LFS [31]. However, most of these LFS methods and products are just based on the immune recognition principles, implying the existence of antibody of targets being the prerequisite. And then, some nucleic acid templates were adopted as the target of LFS analysis [32, 33], and satisfied results were achieved even for on-site COVID-19 diagnosis [34, 35]. Intrinsically, these nucleic acid detections are the analysis of the target template by direct hybridization, and the detection limit of these methods without amplification are far away from the practical requirements.
In this study, the extraordinary amplification capability of PCR was deftly integrated with simplicity of LFS for rapid and easy identification of HPV target genotypes. The functionalized amplicons of HPV-16 and HPV-18 were simultaneously produced by the portable thermal controller with the designed functional primer sets. Then, the amplicons of both HPV genotypes were visually judged with the designed LFS. And the high-risk genotypes of HPV-16 and 18 clinical samples were successfully identified by this molecular LFS without any assistance of expensive hardware.
Experimental
Chemicals and apparatus
The conjugate pads, sample pads, polyvinyl chloride (PVC) adhesive backing pads, absorption pads, and nitrocellulose membranes were all purchased from Jie-ning Biotech. Co. Ltd. (Shanghai, China). Guillotine cutting module CM4000 and XYZ 3000 Dispensing Platform were purchased from Bio-Dot Inc. (Irvine, CA, USA). Taq DNA polymerase (5 U/μL), dNTP mix (25 mM), DNA ladder, 4S Red Plus nucleic acid stain (1000 ×), streptavidin (SA), and agarose were all obtained from Sangon Biotech (Shanghai, China). Chloroauric acid tetrahydrate (HAuCl4·4H2O) was purchased from J&K Co. Ltd. (Shanghai, China). Bovine serum albumin (BSA) was purchased from Bio-Dee Biotech. Co. Ltd. (Beijing, China). Fluorescein isothiocyanate (FITC) antibody and digoxin (DIG) antibody were purchased from Sinopharm Chemical (Wuhan, China). Other common reagents used in this study were all purchased from Sinopharm Chemical Reagent Co., Ltd. (Wuhan, China) of analytical grade. Ultrapure deionized water (> 18 MΩ·cm) was used throughout the test. The one-step nucleic acid release and storage buffer was purchased from the Shenzhen E-bio Intelligence & Biotechnology Company (Shenzhen, China).
All HPV clinical samples used in this research were obtained from the First Affiliated Hospital of Anhui Medical University and all the studies conducted have been approved by the ethic committee of Anhui Medical University (P-2021–18-25). For optimizing the detection conditions of designed protocol and confirming the specificity and sensitivity of designed primer sets, recombination plasmids that contain HPV-16 and 18 DNA, respectively, were synthesized by Sangon Biotech (Shanghai, China, www.sangon.com).
Primer set design and functionalization
The E6 and E7 open reading frames of HPV-16 and 18 were downloaded from GenBank and aligned (GenBank Accession Numbers K02718 and X05015, respectively). Species-specific primer sets for HPV-16 and 18 were designed by primer premier 5.0, respectively, and synthesized from General Biosystems (Anhui, China). The specificity of two pairs of primers was verified by the BLAST program. The detailed sequences of the designed primer sets were given and could be checked in Table S1. Typically, in order to realize simultaneous amplification of both genotypes and production of functional amplicons for rapid identification with LFS, each primer set should be carefully designed and functionalized without any cross-reactivity.
Simple treatment of clinical samples for rapid and effective DNA extraction
For wide application in the resource-limited settings, simple extraction treatments of samples were the first choice and one-step direct lysis protocol was adopted and optimized. Typically, the nucleic acid lysis and release reagent was added into the clinical collected samples (cervical exfoliated cells) at the volume ratio of 1:3, and the reaction mixture was kept for 5 min at room temperature with rotation, and then the mixture could be used directly for amplification. Compared with the traditional MNP-based method [36, 37] for nucleic acid extraction, this method holds the characteristics of rapidity, simplicity, and good repeatability without the need of magnetic beads, magnetic stand, and multi-step operations. Of great significance, this direct lysis protocol can be adopted for the treatment of the collected cervical exfoliated cells samples by the swab and can be conducted at home or in the resource-limited settings in less than 5 min.
Preparation of AuNPs and AuNP-FITC antibody conjugates
AuNPs were prepared based on the classic reduction of gold chloride with trisodium citrate using a previous reported method with minor modifications [38, 39] and the detailed preparation procedures and the characterization results of AuNPs and AuNP-FITC antibody conjugates can be found in the supporting information (Figure S1).
Construction of the molecular lateral flow strip
Before the assembly and construction of molecular LFS for detection of amplicons, the sample pad was saturated overnight with a buffer containing 50 mM Tris–HCl (pH 8.0), 150 mM NaCl, and 0.25% Triton X-100, while the conjugate pad was pretreated by a buffer containing 10 mM PB (pH 7.4), 5% sucrose, 1% trehalose, 0.3% Tween-20, and 0.25% PEG 20,000. Then, 6 μL of the prepared AuNP-FITC antibody conjugates is spotted onto the conjugate pad by the Biodot XYZ 3000. All treated components were dried at 30 °C for future use. Five components including a sample pad, a conjugate pad, a nitrocellulose (NC) membrane, an absorbent pad, and a plastic adhesive pad are assembled to construct the LFS, as depicted in Fig. 1 (II). For the preparation of NC membrane, typically, DIG antibody and SA were immobilization onto the NC membrane as the test line 1 (T1 line) and test line 2 (T2 line) corresponding to the HPV-18 and HPV-16, respectively, while the goat-anti-mouse antibody was immobilized as the control line (C line). For assembly the LFS, it should be noted that each pad was overlapped with adjacent pads with a length of 2 mm to ensure the successful migration of the detection fluids.
Simultaneous amplification of HPV-16 and HPV-18 with portable PCR
Both HPV-16 and HPV-18 were simultaneously amplified in the same system. The amplifications of this duplex PCR were carried out in the 50 μL reaction volume containing 1 × PCR buffer (10 mM Tris–HCl (pH 8.8), 50 mM KCl, 0.08% (v/v) NP-40), 160 nM of primer set of 16-F/16-R, 80 nM of primer set of 18-F/18-R, 0.2 mM of each dNTP, 2 mM MgCl2, 1.5 U Taq DNA polymerase, and 1 μL of DNA template. PCR amplifications were performed with the portable thermal controller (Hangzhou Aomin Biological Ltd. Co.) under the following conditions: denatured at 95 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 20 s, annealing at 58 °C for 20 s and extension at 72 °C for 20 s followed by a final extension incubation for 3 min at 72 °C. Negative control reaction mixture contained the ultrapure deionized water instead of extracted HPV genomic templates. About 5 μL PCR product was confirmed by 2% (w/v) agarose gel electrophoresis in 1 × TBE buffer at a constant voltage of 200 V for 20 min.
Simultaneous visual detection of HPV-16 and 18 with the designed molecular LFS
For rapid analysis of the obtained mixture of functional amplicons, the products were diluted five times with 10 mM PB (pH 7.0) and loaded directly onto the sample pad of LFS. The signals on T1 line, T2 line, and C line can be visually judged and further quantitatively analyzed with ImageJ and the infection of HPV-16 or HPV-18 can be easily diagnosed without any complicated operations.
The collected HPV clinical samples were the cervical exfoliated cells and obtained from the First Affiliated Hospital of Anhui Medical University, which were all pre-determined with real-time PCR. The obtained samples were treated with the direct lysis and extraction reagents and the mixtures were used directly for amplification. All detection results detected with our molecular LFS were compared with those of real-time PCR.
Results and discussion
Mechanism for simultaneous visual judgement of HPV-16 or 18 infection
Although identification HPV-16 or HPV-18 infection can be well realized with the current molecular protocols in professional labs, rapid and easy screening of HPV-16/HPV-18 without precise instruments or professional operators are still urgently needed in the developing countries, especially in the resource-limited settings. In this research, firstly, rapid gene extraction treatment was studied for easy operations. Then, the on-site amplification with portable thermal controller was integrated with the LFS to achieve the simultaneous visual and easy identification of different amplicons. The designed schematic diagram of the research was shown in Fig. 1 (I). With the designed primer sets for both HPV-16 (FITC and biotin labeled primer set) and HPV-18 (FITC and DIG-labeled primer set), in the presence of target viral genotypes, the sequences can be amplified with the portable thermal controller, respectively, and dual functionalized amplicons of HPV-16/HPV-18 can be obtained. FITC acts as the universal tag, which can be recognized by the AuNP-FITC Ab. The biotin and DIG are the specific tags for HPV-16/HPV-18, which can be recognized and captured by the SAV on T2 line and DIG antibody on T1 line, respectively. The formed recognition structures (AuNP-FITC-dsDNA-SAV on T2 line and AuNP-FITC-dsDNA-DIG on T1 line) and the captured AuNPs on different test lines induce the observable optical signal. Therefore, different amplicons can be directly measured with the designed LFS and HPV-16/HPV-18 can be visually differentiated by the distinct T2 line and T1 line on LFS. On the contrary, without HPV-16 and HPV-18 templates, there will be no formation of recognition induced structures on T1 or T2 line and no capturing of AuNPs on test lines, showing no observable optical signals on test lines (Fig. 1 (II)). Of great significance, simultaneous detection of both HPV-16 and HPV-18 will not be influenced by the detection results of each other even one of them is at the comparable trace amount. Meanwhile, for the validation of each test, whether the existence of HPV-16/HPV-18 or not, the immobilized secondary antibody on C line can recognize the goat-anti-mouse antibody on AuNPs and capture the AuNPs on C line, showing the observable red color of C line and demonstrating the validation of the detection.
Feasibility of one-step nucleic acid extraction
In order to well meet the requirements for easy identification of high-risk HPV genotypes in the resource-limited settings, the pretreatment of the cell samples for gene extraction is also of critical significance. The direct lysis protocol was the first consideration for on-site applications. The pre-confirmed negative, HPV-16 and HPV-18 positive samples were treated directly with our lysis protocol. The obtained DNA of HPV-16 and HPV-18 has been measured with the nucleic acid quantification instrument, and the concentration of HPV-16, HPV-18 was 501.49 ng/Μl and 438.5 ng/μL, respectively. Then, these obtained genomic materials are adopted as the target temples for amplification, and the detection results are demonstrated in Fig. 2. For one aspect, the results of agarose gel electrophoresis in the left image of Fig. 2 indicate that the genetic materials can be effectively extracted and amplified with PCR proved by the presence of target bands in the HPV-16 and HPV-18 positive lanes, respectively. For the other, results in the right image of Fig. 2 also demonstrate that the amplified products of both HPV-16 and HPV-18 can be well measured with our designed molecular LFS and the positive signals of HPV-16 or HPV-18 can be well judged by visual observation and distinguished from each other.
Feasibility verification results. Left image: illustration of agarose gel electrophoresis (AGE) verification (M: maker; 1: blank control; 2: negative sample; 3: HPV-16 positive sample; 4: blank control; 5: negative sample; 6: HPV-18 positive sample). Right image: results of LFS verification, the first three LFS were used to detect the HPV-16, and the last three LFS were used to detect the HPV-18. (FPHPV18: forward primer of HPV-18; RPHPV18: reverse primer of HPV-18; FPHPV16: forward primer of HPV-16; RPHPV16: reverse primer of HPV-16; THPV18: template of HPV-18; THPV16: template of HPV-16. Green circle means with the presence of the component; empty circle means without the component)
Optimization of conditions for the identification of HPV-16/HPV-18
In order to achieve the best detection performance of designed protocol for rapid and simultaneous identification of both HPV-16 and HPV-18 in clinical samples, some key parameters of both the amplification and LFS measurements were optimized. The concentrations of primer set of HPV-16 and HPV-18 were optimized as 160 nM and 80 nM, respectively (see optimized results in Figure S2 and discussions in Supporting Information). The LFS for rapid analysis of produced amplicons were also carefully studied and optimized. 1 mg/mL of DIG antibody on T1 line, 1.2 mg/mL SA on T2 line, and 6 μL of AuNP-FITC antibody conjugates on the conjugation pad were adopted as the optimal conditions for the detection with LFS. Besides, the dilution of amplicons for LFS detection was optimized and 8 μL amplicon mixed with 40 μL 10 mM PB (pH 7.0) was treated as the best condition for the visual measurements, which could guarantee the optical signals on both Tl and T2 lines for visual observation (see detailed results in Figure S3).
Analytical performance of this constructed strategy
Under above optimized amplification and visual measurement conditions, target HPV-16 and HPV-18 DNA at different concentrations were detected. The samples were prepared with both HPV-16 and HPV-18 DNA at different spike ratios. From results shown in Fig. 3, in the samples from left to right, the concentration of HPV-18 DNA is increased, while the concentration of HPV-16 is decreased accordingly. The agarose gel electrophoresis results clearly demonstrate that the optical intensity of bands corresponding to HPV-16 (the lower bands) are decreased while that of HPV-18 (the upper bands) increased, which are in accordance with concentration variation trends. And the detection limit of both HPV-16 and HPV-18 could come to 0.7 × 103 copies. Of great significance, the visual measured results with LFS are in total agreement with those of agarose gel electrophoresis. And the lowest detection limits of electrophoresis are also as low as 0.7 × 103 copies for both HPV-16 and HPV-18 genotypes. Although, for this achieved detection limit, it is comparable to that of the electrophoresis or better than other normal molecular protocols (see comparison results in Table 1) and can be adopted for qualitative screening, for practical applications, the detection limit should be the more sensitive the better and further improvement of the detection limit to the final single-cell resolution for better performance is still of significance. In that case, trace target high-risk cells can be accurately screened as early as possible. Besides, it could also be found in Fig. 3 that simultaneous detection of both genotypes does not interfere with each other. Comparatively speaking, this molecular LFS can be the ideal alternative protocol of the traditional PCR and even comparable to the real-time fluorescent PCR with simpler operations and lower cost. Meanwhile, different concentrations of both HPV-16 and HPV-18 can be qualitatively judged by naked-eye observation, which can meet the requirements of practical screening of high-risk HPV. For further quantitative analysis, the results of LFS can be read and measured directly by the photos taken with designed apps. The interference of ambient brightness and other conditions can be effectively avoided by the standard color and inbuilt QR code, which would be acquired simultaneously by taking photos. The semi-quantitative analysis could be achieved by the calibration curve information that the QR code included. Related studies are still ongoing in our labs.
Illustration of the designed protocol’s sensitivity: the results at different concentrations of HPV-16 and HPV-18; left panel: results of agarose gel electrophoresis to decreasing concentrations of HPV-16 and increasing concentrations of HPV-18; right panel: results of LFS to decreasing concentrations of HPV-16 and increasing concentrations of HPV-18; and the quantitative analysis of the LFS results with ImageJ
Furthermore, the specificity of this designed amplification-assisted molecular LFS protocol for rapid and simultaneous identification of HPV-16 and 18 was investigated. Twenty kinds of real-time PCR confirmed HPV genotypes including HPV-16, 18, 6, 11, 30, 31, 33, 35, 39, 44, 45, 51, 52, 53, 56, 58, 59, 66, 68, and 81 were adopted for PCR amplification and analyzed by traditional gel electrophoresis and our amplification-assisted molecular LFS, respectively. Detailed results shown in Fig. 4 demonstrate that with designed primer sets, only the target HPV-16 and HPV-18 can be amplified, and the specific band of the gel can be observed. For other genotypes of HPV detected with designed primer set, there is no band in the gel, indicating the excellent specificity of the designed primer sets. However, the specificity may also be influenced by the subsequent LFS detection. Results in Fig. 4 well demonstrate the specificity of the final LFS detection. Besides the C line on all LFS indicating the validation of detection, only the three groups of HPV-16/HPV-18, HPV-16, and HPV-18 show the observable signals on the T lines (T1 and T2 line for HPV-16/HPV-18; T2 line for HPV-16; T1 line for HPV-18). Other genotypes of HPV do not show any signals on test lines of molecular LFS. This extraordinary specificity of the designed protocol is attributed to the excellent specificity of two designed primer sets for HPV-16 and 18, respectively. Meanwhile, the specific recognition systems including the FTIC, DIG, and biotin also contribute to this excellent specificity.
Finally, the amplification-assisted molecular LFS was utilized for the rapid visual identification of HPV-16 and HPV-18 clinical cell samples. Twenty real-time PCR confirmed clinical cell samples were treated and detected with our amplification-assisted molecular LFS. All visual detection results of LFS are shown in Fig. 5. From the visual judged results, all the HPV-16 or HPV-18 positive samples have been labeled with the symbol of star (*). There are 2 samples (samples 1 and 6) simultaneously infected by high-risk 16 and 18 genotypes, while other 5 samples (sample 4, 7, 12, 16, and 18) are infected by just one genotype of HPV-16 or 18. Meanwhile, two important aspects should be noted: (1) Both the HPV-16 & HPV-18 can be well identified and distinguished from each other (4 and 18 of HPV-16 while 7, 12, and 16 of HPV-18) with the sample amplification-assisted molecular LFS; (2) The severe and mild infections can be well differentiated of the same genotype (12 of mild infection and 16 of severe infection of HPV-18). And these visual judgments obtaining positive results are totally in agreement with the pre-confirmed results of real-time PCR, demonstrating the great capability of this amplification-assisted molecular LFS for rapid and visual identification of high-risk HPV genotypes. Besides, one thing should be noted that although two genotypes have been successfully identified simultaneously on the same LFS, there are other 10 high-risk genotypes required for identification. For the same LFS, the max number of analytes would be no more than 5 or 6 genotypes due to the limited physical length of the lateral flow strip. In the future studies, some multi-LFS can be combined together to realize the whole high-risk genotypes screening of HPV. Alternatively, the functional primer sets can be mixed and the recognition systems can also be mixed on the single test line. If positive results obtained on the single test line, the new round screening with multi test lines on the new LFS will be conducted for confirmation of exact genotypes. And this multi-round screening can well resolve the high throughput identification of high-risk HPV genotypes. Furthermore, the systematic integration of amplification with the LFS can realize the “sample in”/ “results out” detection, which greatly contributes to the easy operation and broaden the application of molecular LFS.
Conclusions
An on-site amplification-assisted molecular LFS strategy has been developed for rapid and visual identification of the high-risk genotypes of HPV for application in the resource-limited settings. To realize the rapid and on-site identification of high-risk HPV-16 and HPV-18; the direct lysis treatments of samples were investigated to adapt the requirement of complicate instrument-free identifications. With the designed functional primer sets, target templates of HPV-16 and HPV-18 can be simultaneously amplified in the same system, and the functional amplicons can be directly measured with the designed molecular LFS. Both HPV-16 and HPV-18 can be simultaneously distinguished from each other by the visible signals on the different test lines on the same LFS. And the clinical samples have been well identified with the satisfied differentiation results of HPV-16 and HPV-18. What should be noted is that all the rapid identification of the high-risk genotypes from the cervical exfoliated cells can be performed in the common labs or even at home without any precise-unmovable instruments by the normal operators or even the consumers themselves. Further effective replacement of PCR with the isothermal amplification strategy such as the recombinase polymerase amplification (RPA) will further empower the molecular LFS without hardware, and related studies are still ongoing in our lab. These are of great importance for the routine screenings of “Two cancers” for guaranteeing the women health in the remote districts and resource-limited settings. Even more, this designed amplification-assisted molecular LFS can be extended to rapid and visual screening of other high-risk genotypes of HPV and diagnosis of other infectious diseases.
References
Castle PE, Einstein MH, Sahasrabuddhe VV (2021) Cervical cancer prevention and control in women living with human immunodeficiency virus. CA-Cancer J Clin 71(6):505–526. https://doi.org/10.3322/caac.21696
Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189(1):12–19. https://doi.org/10.1002/(sici)1096-9896(199909)189:1%3c12::aid-path431%3e3.0.co;2-
Xu HH, Zheng LZ, Lin AF, Dong SS, Chai ZY, Yan WH (2018) Human papillomavirus (HPV) 18 genetic variants and cervical cancer risk in Taizhou area, China. Gene 647:192–197. https://doi.org/10.1016/j.gene.2018.01.037
Zhai L, Yadav R, Kunda NK, Anderson D, Bruckner E, Miller EK, Basu R, Muttil P, Tumban E (2019) Oral immunization with bacteriophage MS2-L2 VLPs protects against oral and genital infection with multiple HPV types associated with head & neck cancers and cervical cancer. Antivir Res 166:56–65. https://doi.org/10.1016/j.antiviral.2019.03.012
Mix J, Saraiya M, Hallowell BD, Befano B, Cheung LC, Unger ER, Gargano JW, Markowitz LE, Castle PE, Raine-Bennett T, Walker J, Zuna R, Schiffman M, Wentzensen N, Gage JC (2022) Cervical precancers and cancers attributed to HPV types by race and ethnicity: implications for vaccination, screening, and management. J Natl Cancer I https://doi.org/10.1093/jnci/djac034
Arbyn M, Ronco G, Anttila A, Meijer CJ, Poljak M, Ogilvie G, Koliopoulos G, Naucler P, Sankaranarayanan R, Peto J (2012) Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 30:F88–F99. https://doi.org/10.1016/j.vaccine.2012.06.095
Arbyn M, Snijders PJ, Meijer CJ, Berkhof H, Cuschieri K, Kocjan BJ, Poljak M (2015) Which high-risk HPV assays fulfil criteria for use in primary cervical cancer screening? Clin Microbiol Infect 21(9):817–826. https://doi.org/10.1016/j.cmi.2015.04.015
Higginson J, DeVita VT (1980) IARC monographs on the evaluation of carcinogenic risk of chemicals to humans. J Clin Pathol 41 (5):passim-A26, A28, A30 passim. https://doi.org/10.1136/jcp.48.7.691-a
Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, Ghissassi FE, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L (2009) A review of human carcinogens—part B: biological agents. Lancet Oncol 10(4):321–322. https://doi.org/10.1016/s1470-2045(09)70096-8
Basu P, Joshi S, Sankaranarayanan R (2015) Human papillomavirus (HPV) testing for secondary prevention of cervical cancer. Curr Obstet Gynecol 4(4):1–12. https://doi.org/10.1007/s13669-015-0133-z
Poljak M, Cuzick J, Kocjan BJ, Iftner T, Dillner J, Arbyn M (2012) Nucleic acid tests for the detection of alpha human papillomaviruses. Vaccine 30(Suppl 5):F100–F106. https://doi.org/10.1016/j.vaccine.2012.04.105
Choi YD, Jung WW, Nam JH, Choi HS, Park CS (2005) Detection of HPV genotypes in cervical lesions by the HPV DNA chip and sequencing. Gynecol oncol 98(3):369–375. https://doi.org/10.1016/j.ygyno.2005.04.044
Ratnam S, Coutlee F, Dan F, Bentley J, Escott N, Ghatage P, Gadag V, Holloway G, Bartellas E, Kum N (2010) Aptima HPV E6/E7 mRNA test is as sensitive as hybrid capture 2 assay but more specific at detecting cervical precancer and cancer. J Clin Microbiol 49(2):557–564. https://doi.org/10.1128/jcm.02147-10
De CP, Coste J, Sastre-Garau X, Thioux M, Bouillac C, Labbé S, Cartier I, Ziol M, Dosda A, Le GC (2003) Efficiency of the hybrid capture 2 HPV DNA test in cervical cancer screening. A study by the French Society of Clinical Cytology. Am J Clin Pathol 120(4):492–499. https://doi.org/10.1309/xfuc-pp6m-5xua-94b8
Weyn C, Boulenouar S, Mathys V, Vanhoolandt J, Bernis A, Fontaine V (2007) Detection of human papillomavirus types 45 and 51 by type-specific polymerase chain reaction. J Virol Methods 146(1):405–408. https://doi.org/10.1016/j.jviromet.2007.08.003
Lugo-Trampe A, Trujillo-Murillo KdC, Rodriguez-Sanchez IP, Barboza-Cerda MdC, Lugo-Trampe JdJ, Hernandez-Ramirez LC, Canseco-Avila LM, Espinoza-Ruiz M, Dominguez-Arrevillaga S, Delgado-Enciso I (2013) A PCR-RFLP method for typing human papillomavirus type 16 variants. J Virol Methods 187(2):338–344. https://doi.org/10.1016/j.jviromet.2012.10.013
Kim CJ, Jeong JK, Park M, Park TS, Park TC, Namkoong SE, Park JS (2003) HPV oligonucleotide microarray-based detection of HPV genotypes in cervical neoplastic lesions. Gynecol Oncol 89(2):210–217. https://doi.org/10.1016/s0090-8258(02)00069-0
Gemignani F, Landi S, Chabrier A, Smet A, Zehbe I, Canzian F, Tommasino M (2004) Generation of a DNA microarray for determination of E6 natural variants of human papillomavirus type 16. J Virol Methods 119(2):95–102. https://doi.org/10.1016/j.jviromet.2004.02.018
Gao G, Chernock RD, Gay HA, Thorstad WL, Zhang TR, Wang H, Ma XJ, Luo Y, Lewis JS Jr, Wang X (2013) A novel RT-PCR method for quantification of human papillomavirus transcripts in archived tissues and its application in oropharyngeal cancer prognosis. Int J Cancer 132(4):882–890. https://doi.org/10.1002/ijc.27739
Wu Q, Yao L, Qin PZ, Xu JG, Sun X, Yao BB, Ren F, Chen W (2021) Time-resolved fluorescent lateral flow strip for easy and rapid quality control of edible oil. Food Chem 357 https://doi.org/10.1016/j.foodchem.2021.129739
Sun Y, Qin PZ, He J, Li WW, Shi YL, Xu JG, Wu Q, Chen QQ, Li WD, Wang XX, Liu GD, Chen W (2022) Rapid and simultaneous visual screening of SARS-CoV-2 and influenza virufses with customized isothermal amplification integrated lateral flow strip. Biosens Bioelectron 197 https://doi.org/10.1016/j.bios.2021.113771
Yao L, Ren LL, Chen Q, Wu YH, Xu JG, Xia Q, Zhang C, Chen W (2022) Rapid and direct concentration range judgment of lamotrigine in plasma by the multi test lines with different detection limits on the same lateral flow strip. Anal Chim Acta 1192:339347–339347. https://doi.org/10.1016/j.aca.2021.339347
Tripathi P, Kumar A, Sachan M, Gupta S, Nara S (2020) Aptamer-gold nanozyme based competitive lateral flow assay for rapid detection of CA125 in human serum. Biosens Bioelectron 165 https://doi.org/10.1016/j.bios.2020.112368
Xiong E, Jiang L, Tian T, Hu M, Yue H, Huang M, Lin W, Jiang Y, Zhu D, Zhou X (2021) Simultaneous dual-gene diagnosis of SARS-CoV-2 based on CRISPR/Cas9-mediated lateral flow assay. Angew Chem Int Edit 60(10):5307–5315. https://doi.org/10.1002/anie.202014506
Ji T, Xu X, Wang X, Cao N, Han X, Wang M, Chen B, Lin Z, Jia H, Deng M, Xia Y, Guo X, Lei M, Liu Z, Zhou Q, Chen G (2020) Background-free chromatographic detection of sepsis biomarker in clinical human serum through near-infrared to near-infrared upconversion immunolabeling. ACS Nano 14(12):16864–16874. https://doi.org/10.1021/acsnano.0c05700
Wu R, Zhou S, Chen T, Li J, Shen H, Chai Y, Li LS (2018) Quantitative and rapid detection of C-reactive protein using quantum dot-based lateral flow test strip. Anal Chim Acta 1008:1–7. https://doi.org/10.1016/j.aca.2017.12.031
Yao L, Teng J, Zhu M, Zheng L, Zhong Y, Liu G, Xue F, Chen W (2016) MWCNTs based high sensitive lateral flow strip biosensor for rapid determination of aqueous mercury ions. Biosens Bioelectron 85:331–336. https://doi.org/10.1016/j.bios.2016.05.031
Cai P, Wang R, Ling S, Wang S (2021) A high sensitive platinum-modified colloidal gold immunoassay for tenuazonic acid detection based on monoclonal IgG. Food Chem 360 https://doi.org/10.1016/j.foodchem.2021.130021
Yang H, Wang Y, Liu S, Ouyang H, Lu S, Li H, Fu Z (2021) Lateral flow assay of methicillin-resistant Staphylococcus aureus using bacteriophage cellular wall-binding domain as recognition agent. Biosens Bioelectron 182 https://doi.org/10.1016/j.bios.2021.113189
Fabiani L, Mazzaracchio V, Moscone D, Fillo S, De Santis R, Monte A, Amatore D, Lista F, Arduini F (2022) Paper-based immunoassay based on 96-well wax-printed paper plate combined with magnetic beads and colorimetric smartphone-assisted measure for reliable detection of SARS-CoV-2 in saliva. Biosens Bioelectron 200:113909–113909. https://doi.org/10.1016/j.bios.2021.113909
Eum NS, Yeom SH, Kwon DH, Kim HR, Kang SW (2010) Enhancement of sensitivity using gold nanorods—antibody conjugator for detection of E. coli O157:H7. Sensor Actuat B-Chem 143(2):784–788. https://doi.org/10.1016/j.snb.2009.09.054
Zheng WL, Yao L, Teng J, Yan C, Qin PZ, Liu GD, Chen W (2018) Lateral flow test for visual detection of multiple MicroRNAs. Sensor Actuat B-Chem 264:320–326. https://doi.org/10.1016/j.snb.2018.02.159
Takalkar S, Baryeh K, Liu G (2017) Fluorescent carbon nanoparticle-based lateral flow biosensor for ultrasensitive detection of DNA. Biosens Bioelectron 98:147–154. https://doi.org/10.1016/j.bios.2017.06.045
Joung J, Ladha A, Saito M, Kim N-G, Woolley AE, Segel M, Barretto RPJ, Ranu A, Macrae RK, Faure G, Ioannidi EI, Krajeski RN, Bruneau R, Huang M-LW, Yu XG, Li JZ, Walker BD, Hung DT, Greninger AL, Jerome KR, Gootenberg JS, Abudayyeh OO, Zhang F (2020) Detection of SARS-CoV-2 with SHERLOCK One-Pot Testing. New Engl J Med 383(15):1492. https://doi.org/10.1056/nejmc2026172
Patchsung M, Jantarug K, Pattama A, Aphicho K, Suraritdechachai S, Meesawat P, Sappakhaw KN, Ruenkam T, Wongsatit T, Athipanyasilp N, Eiamthong B, Lakkanasirorat B, Phoodokmai T, Niljianskul N, Pakotiprapha D, Chanarat S, Homchan A, Tinikul R, Kamutira P, Phiwkaow K, Soithongcharoen S, Kantiwiriyawanitch C, Pongsupasa V, Trisrivirat D, Jaroensuk J, Wongnate T, Maenpuen S, Chaiyen P, Kamnerdnakta S, Swangsri J, Chuthapisith S, Sirivatanauksorn Y, Chaimayo C, Sutthent R, Kantakamalakul W, Joung J, Ladha A, Jin X, Gootenberg JS, Abudayyeh OO, Zhang F, Horthongkham N, Uttamapinant C (2020) Clinical validation of a Cas13-based assay for the detection of SARS-CoV-2 RNA. Nat Biomed Eng 4(12):1140–1149. https://doi.org/10.1038/s41551-020-00603-x
Zhao Y, Hao C, Yong Q, Qu C, Chen W, Peng C, Kuang H, Zhou H, Wang L, Xu C (2012) Systematic comparisons of genetically modified organism DNA separation and purification by various functional magnetic nanoparticles. Int J Food Sci Tech 47(5):910–917. https://doi.org/10.1111/j.1365-2621.2011.02921.x
Qiao DQ, Xu JG, Qin PZ, Yao L, Lu JF, Eremin S, Chen W (2018) Highly simple and sensitive molecular amplification-integrated fluorescence anisotropy for rapid and on-site identification of adulterated beef. Anal Chem 90(12):7171–7175. https://doi.org/10.1021/acs.analchem.8b01374
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys 241(105):20–22. https://doi.org/10.1038/physci241020a0
Roth J (1982) The preparation of protein A-gold complexes with 3 nm and 15nm gold particles and their use in labelling multiple antigens on ultra-thin sections. Histochem J 14(5):791–801. https://doi.org/10.1007/BF01033628
Rodriguez NM, Wong WS, Liu L, Dewar R, Klapperich CM (2016) A fully integrated paperfluidic molecular diagnostic chip for the extraction, amplification, and detection of nucleic acids from clinical samples. Lab Chip 16(4):753–763. https://doi.org/10.1039/c5lc01392e
Kuo YB, Li YS, Chan EC (2015) Rapid identification of HPV 16 and 18 by multiplex nested PCR-immunochromatographic test. J Virol Methods 212:8–11. https://doi.org/10.1016/j.jviromet.2014.10.009
Tsou JH, Leng Q, Jiang F (2019) A CRISPR test for detection of circulating nuclei acids. Transl Oncol 12(12):1566–1573. https://doi.org/10.1016/j.tranon.2019.08.011
Kim SJ, Nahm KB, Lim JB, Oh SW, Choi EY (2014) A rapid and sensitive detection of HPV by combined assay of PCR and fluorescence DNA chip. Biochip J 8(1):48–54. https://doi.org/10.1007/s13206-014-8108-0
Funding
This work is financially supported by the grant of the NSFC (32172295, 21804028), the project of Shanghai Municipal Science and Technology Commission (22N31900500), the fund of Intelligent Manufacturing Institute of HFUT (IMICZ2019007), the China Postdoctoral Science Foundation (2019M652167), the postdoc grant of Anhui (2020B412) the Young and Middle-aged Leading Scientists, Engineers and Innovators of the XPCC (No. 2019CB017), and the China Agriculture Research System-48 (CARS-48).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Q., Yao, L., Wu, Q. et al. Rapid and simultaneous visual typing of high-risk HPV-16/18 with use of integrated lateral flow strip platform. Microchim Acta 189, 350 (2022). https://doi.org/10.1007/s00604-022-05449-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05449-5