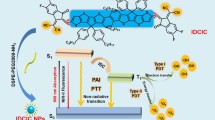
Abstract
Although upconversion photodynamic therapy (PDT) has gained extensive interests in disease treatment, the intracellular migration pathway of upconversion photosensitizers and underlying cell-particle interaction mechanism is still largely unexplored. In this work photoswitchable upconversion nanoparticles (UCNPs) are reported that can release orthogonal emissions excited by two near-infrared lights, i.e., red color of 980-nm and green color of 808-nm light excitation. Taking advantage of the dual-emissive property, a methodology based on Pearson’s correlation analysis is proposed to verify the accuracy of upconversion luminescence signals under different excitation lights, which has been previously neglected. Meanwhile, we have designed a near-infrared mediated bioimaging nanoplatform that can generate reactive oxygen species (ROS) using one light and simultaneously track the location of upconversion photosensitizers using another excitation light. Our study not only depicts the migration pathway of upconversion photosensitizers, but also demonstrates the organelle escape of these upconversion nanoparticles via PCI (photochemical internalization) process. It is believed that our results inspire more efficient synergistic therapy by combining PDT with other modalities in a programmable manner.
Graphical abstract









Similar content being viewed by others
References
Hopper C (2000) Photodynamic therapy: a clinical reality in the treatment of cancer. Lancet Oncol 1:212–219. https://doi.org/10.1016/S1470-2045(00)00166-2
Huang Z (2005) A review of progress in clinical photodynamic therapy. Technol Cancer Res Treat 4:283–293. https://doi.org/10.1177/153303460500400308
Zhu X, Zhang J, Liu J, Zhang Y (2019) Recent progress of rare-earth doped upconversion nanoparticles: synthesis, optimization, and applications. Adv Sci 6:1901358. https://doi.org/10.1002/advs.201901358
Li Q, Yuan S, Liu F et al (2021) Lanthanide-doped nanoparticles for near-infrared light activation of photopolymerization: fundamentals, optimization and applications. Chem Rec 21:1681–1696. https://doi.org/10.1002/tcr.202100093
Sheng T, Xu M, Li Q et al (2021) Elucidating the role of energy management in making brighter, and more colorful upconversion nanoparticles. Mater Today Phys 20:100451. https://doi.org/10.1016/j.mtphys.2021.100451
Wang J, Sheng T, Zhu X et al (2021) Spectral engineering of lanthanide-doped upconversion nanoparticles and their biosensing applications. Mater Chem Front 5:1743–1770. https://doi.org/10.1039/D0QM00910E
Zhang Y, Lei P, Zhu X, Zhang Y (2021) Full shell coating or cation exchange enhances luminescence. Nat Commun 12:6178. https://doi.org/10.1038/s41467-021-26490-7
Shao Y, Liu B, Di Z et al (2020) Engineering of upconverted metal-organic frameworks fornear-infrared light-triggered combinational photodynamic/chemo-/immunotherapy against hypoxic tumors. J Am Chem Soc 142:3939–3946. https://doi.org/10.1021/jacs.9b12788
Dong S, Xu J, Jia T et al (2019) Upconversion-mediated ZnFe2O4 nanoplatform for NIR-enhanced chemodynamic and photodynamic therapy. Chem Sci 10:4259–4271. https://doi.org/10.1039/c9sc00387h
Li G, Wang W, Song S et al (2019) Anticancer effects and cell death pathways in ultralow-Power 980 nm laser-triggered photodynamic therapy by Gd2 O3:Yb, Tm nanoparticles. J Biomed Nanotechnol 15:462–476. https://doi.org/10.1166/jbn.2019.2702
Zhang Z, Jayakumar MKG, Zheng X et al (2019) Upconversion superballs for programmable photoactivation of therapeutics. Nat Commun 10:4586. https://doi.org/10.1038/s41467-019-12506-w
Tang M, Zhu X, Zhang Y et al (2019) Near-infrared excited orthogonal emissive upconversion nanoparticles for imaging-guided on-demand therapy. ACS Nano 13:10405–10418. https://doi.org/10.1021/acsnano.9b04200
Chen G, Wu Y, Jin K et al (2021) A biosynthesized near-infrared-responsive nanocomposite biomaterial for antimicrobial and antibiofilm treatment. ACS Appl Bio Mater 4:7542–7553. https://doi.org/10.1021/acsabm.1c00790
Yang M, Wang H, Wang Z et al (2019) A Nd3+ sensitized upconversion nanosystem with dual photosensitizers for improving photodynamic therapy efficacy. Biomater Sci 7:1686–1695. https://doi.org/10.1039/c8bm01570h
Ding B, Shao S, Yu C et al (2018) Large-pore mesoporous-silica-coated upconversion nanoparticles as multifunctional immunoadjuvants with ultrahigh photosensitizer and antigen loading efficiency for improved cancer photodynamic immunotherapy. Adv Mater 30:1–10. https://doi.org/10.1002/adma.201802479
Zhang Y, Zhu X, Zhang J et al (2021) Synergistic upconversion photodynamic and photothermal therapy under cold near-infrared excitation. J Colloid Interface Sci 600:513–529. https://doi.org/10.1016/j.jcis.2021.05.017
Feng C, Chen L, Lu Y et al (2019) Programmable Ce6 delivery via cyclopamine based tumor microenvironment modulating nano-system for enhanced photodynamic therapy in breast cancer. Front Chem 7:1–11. https://doi.org/10.3389/fchem.2019.00853
Dou QQ, Teng CP, Ye E, Loh XJ (2015) Effective near-infrared photodynamic therapy assisted by upconversion nanoparticles conjugated with photosensitizers. Int J Nanomedicine 10:419. https://doi.org/10.2147/IJN.S74891
Lan Y, Zhu X, Tang M et al (2020) Construction of a near-infrared responsive upconversion nanoplatform against hypoxic tumors via NO-enhanced photodynamic therapy. Nanoscale 12:7875–7887. https://doi.org/10.1039/C9NR10453D
Li L, Zhang P, Yang X et al (2021) Self-assembly of a disulfide-containing core/shell nanocomplex with intracellular environment-sensitive facilitated endo-lysosomal escape for enhanced antitumor efficacy. J Mater Sci 56:4380–4395. https://doi.org/10.1007/s10853-020-05515-4
He K, Zhu J, Gong L et al (2021) In situ self-assembly of near-infrared-emitting gold nanoparticles into body-clearable 1D nanostructures with rapid lysosome escape and fast cellular excretion. Nano Res 14:1087–1094. https://doi.org/10.1007/s12274-020-3153-6
Smith SA, Selby LI, Johnston APR, Such GK (2019) The endosomal escape of nanoparticles: toward more efficient cellular delivery. Bioconjug Chem 30:263–272. https://doi.org/10.1021/acs.bioconjchem.8b00732
Kwon S, Ko H, You DG et al (2019) Nanomedicines for reactive oxygen species mediated approach: an emerging paradigm for cancer treatment. Acc Chem Res 52:1771–1782. https://doi.org/10.1021/acs.accounts.9b00136
Dong K, Wang Z, Zhang Y et al (2018) Metal-organic framework-based nanoplatform for intracellular environment-responsive endo/lysosomal escape and enhanced cancer therapy. ACS Appl Mater Interfaces 10:31998–32005. https://doi.org/10.1021/acsami.8b11972
Miyoshi Y, Kadono M, Okazaki S et al (2020) Endosomal escape of peptide-photosensitizer conjugates is affected by amino acid sequences near the photosensitizer. Bioconjug Chem 31:916–922. https://doi.org/10.1021/acs.bioconjchem.0c00046
Fedorov PP, Aleksandrov VB, Bondareva OS, et al (2001) Concentration dependences of the unit-cell parameters (r= rare-earth elements) 46:280–286
Johnson NJJ, van Veggel FCJM (2014) Lanthanide-based heteroepitaxial core–shell nanostructures: compressive versus tensile strain asymmetry. ACS Nano 8:10517–10527. https://doi.org/10.1021/nn503946t
Tang M, Zhu X, Zhang Y, et al Near-infrared excited orthogonal emissive upconversion nanoparticles for imaging-guided on-demand therapy. ACS Nano 13:10405–10418. https://doi.org/10.1021/acsnano.9b04200
Liu D, Xu X, Du Y et al (2016) Three-dimensional controlled growth of monodisperse sub-50 nm heterogeneous nanocrystals. Nat Commun 7:10254. https://doi.org/10.1038/ncomms10254
Chen Q, Xie X, Huang B et al (2017) Confining excitation energy in Er3+-sensitized upconversion nanocrystals through Tm3+-mediated transient energy trapping. Angew Chemie - Int Ed 56:7605–7609. https://doi.org/10.1002/anie.201703012
Pike JA, Styles IB, Rappoport JZ, Heath JK (2017) Quantifying receptor trafficking and colocalization with confocal microscopy. Methods 115:42–54. https://doi.org/10.1016/j.ymeth.2017.01.005
Zhu YX, Jia HR, Pan GY et al (2018) Development of a light-controlled nanoplatform for direct nuclear delivery of molecular and nanoscale materials. J Am Chem Soc 140:4062–4070. https://doi.org/10.1021/jacs.7b13672
Zinchuk V, Zinchuk O, Okada T (2007) Quantitative colocalization analysis of Mmlticolor confocal immunofluorescence microscopy images: pushing pixels to explore biological phenomena. ACTA Histochem Cytochem 40:101–111. https://doi.org/10.1267/ahc.07002
Lavancier F, Pécot T, Zengzhen L, Kervrann C (2017) A fast automatic colocalization method for 3D live cell and super-resolution microscopy. Hal 1577118. https://hal.inria.fr/hal-01577118
Funding
We acknowledge the financial support from National Natural Science Foundation of China (No. 11905123, 81971740, and 31671011), Shanghai Sailing Program (19YF1415200), and Innovative Research Team of High-Level Local Universities in Shanghai.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiang, Y., Zheng, S., Yuan, S. et al. Near-infrared mediated orthogonal bioimaging and intracellular tracking of upconversion nanophotosensitizers. Microchim Acta 189, 120 (2022). https://doi.org/10.1007/s00604-022-05218-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05218-4