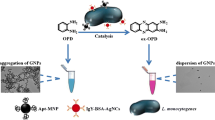
Abstract
A host–guest colorimetric strategy is described for the detection of Listeria monocytogenes (L. monocytogenes). The optical probes were self-assembled based on the supramolecular interactions between the carbonyl groups of cucurbit[7]uril portals and gold nanoparticles (CB[7]-AuNPs). Aptamer and urease modified magnetic nanoparticles were used to specifically recognize and binding to L. monocytogenes, simultaneously hydrolyzing urea to produce ammonium ion (NH4+) that can reverse CB[7] induced AuNPs aggregation. In the presence of L. monocytogenes, the above-mentioned magnetic conjugates preferentially bind to the bacterial surface, which results in blocking the catalytic active sites, thus inhibiting the production of ammonium ions. The normalized absorbance ratio of A700 nm/A525 nm was proportional to the L. monocytogenes concentration ranging from 10 to 106 cfu·mL−1, and the visual determination can be done down to 10 cfu·mL−1. For spiked food samples analyzed without pre-enrichment, recoveries of 98.4% to 99.3% were achieved could be verified and RSD were less than 10%. This work may offer a broad prospect for sensitive and specific determination of pathogens.
Graphical abstract







Similar content being viewed by others
References
Beuchat LR, Doyle MP (2007) Food microbiology: fundamentals and frontiers, third edition || Rapid methods for the detection of foodborne pathogens: current and next-generation technologies. https://doi.org/10.1128/9781555815912: 911-934
Russini V et al (2021) A nosocomial outbreak of invasive listeriosis in an Italian hospital: epidemiological and genomic features. Pathogens 10(5):591
Centers for Disease Control and Prevention (CDC). Listeria (Listeriosis) (2020). Available from: https://www.cdc.gov/listeria/index.html/. (Accessed 29 June 2020)
Farber JM, Peterkin PI (1991) Listeria monocytogenes, a food-borne pathogen. Microbiol Rev 55(3):476–511
Beuselinck K, van Ranst M, van Eldere J (2005) Automated extraction of viral-pathogen RNA and DNA for high-throughput quantitative real-time PCR. J Clin Microbiol 43(11):5541–5546
Cocolin L et al (2005) Analysis of PCR-based methods for characterization of Listeria monocytogenes strains isolated from different sources. Int J Food Microbiol 103(2):167–178
Yanmei H et al (2019) Multicolor and ultrasensitive enzyme-linked immunosorbent assay based on the fluorescence hybrid chain reaction for simultaneous detection of pathogens. J Agric Food Chem 67(33):9390–9398
Garrido-Maestu A et al (2019) Combination of immunomagnetic separation and real-time recombinase polymerase amplification (IMS-qRPA) for specific detection of Listeria monocytogenes in smoked salmon samples. J Food Sci 84(7):1881–1887
Malic L et al (2015) Polymer-based microfluidic chip for rapid and efficient immunomagnetic capture and release of Listeria monocytogenes. Lab Chip 15(20):3994–4007
Durner J (2010) Clinical chemistry: challenges for analytical chemistry and the nanosciences from medicine. Angew Chem Int Ed Engl 49(6):1026–1051
Liu J et al (2015) One-pot synthesis of gold nanoclusters with bright red fluorescence and good biorecognition abilities for visualization fluorescence enhancement detection of E. coli. Talanta 134:54–59
Lopez MM, Atherton AA, Tong WG (2009) Ultrasensitive detection of proteins and antibodies by absorption-based laser wave-mixing detection using a chromophore label. Anal Biochem 399(2):147–151
Sengupta A, Mujacic M, Davis EJ (2006) Detection of bacteria by surface-enhanced Raman spectroscopy. Anal Bioanal Chem 386(5):1379–1386
Resch-Genger U et al (2008) Quantum dots versus organic dyes as fluorescent labels. Nat Methods 5(9):763–775
Daraee H et al (2016) Application of gold nanoparticles in biomedical and drug delivery. Artif Cells Nanomed Biotechnol 44(1):410–422
Chang CC et al (2019) Gold nanoparticle-based colorimetric strategies for chemical and biological sensing applications. Nanomaterials 9(6):861
Jiang Y et al (2017) Aptamer/AuNP biosensor for colorimetric profiling of exosomal proteins. Angew Chem Int Ed Engl 56(39):11916–11920
Mao K et al (2019) Rapid duplexed detection of illicit drugs in wastewater using gold nanoparticle conjugated aptamer sensors. Sci Total Environ 688:771–779
Wu H et al (2020) Host–guest interactions between oxaliplatin and cucurbit[7]uril/cucurbit[7]uril derivatives under pseudo-physiological conditions. Langmuir 36(5):1235–1240
Tonga GY et al (2015) Binding studies of cucurbit[7]uril with gold nanoparticles bearing different surface functionalities. Tetrahedron Lett 56(23):3653–3657
Gao Y et al (2016) CB[7]-mediated signal amplification approach for sensitive surface plasmon resonance spectroscopy. Biosens Bioelectron 81:207–213
Kim C et al (2015) Regulating exocytosis of nanoparticles via host-guest chemistry. Org Biomol Chem 13(8):2474–2479
Giralt M, Buñuel C, Raichs A (2015) Application of the generalized molar-ratio method to the determination of the stoichiometry and apparent binding constant of nanoparticle-organic capping systems. Sangre 27(10):2302–2312
Li H et al (2019) A label-free impedimetric sensor for the detection of an amphetamine-type derivative based on cucurbit[7]uril-mediated three-dimensional AuNPs. Electrochem Commun 100:126–133
Shi X et al (2016) Construction of a graphene/Au-nanoparticles/cucurbit[7]uril-based sensor for Pb(2+) sensing. Chem Eur J 22(16):5643–5648
Sinha S et al (2018) Reversible encapsulations and stimuli-responsive biological delivery from a dynamically assembled cucurbit[7]uril host and nanoparticle guest scaffold. J Mater Chem B 6(44):7329–7334
Fan K et al (2016) Optimization of Fe3O4 nanozyme activity via single amino acid modification mimicking an enzyme active site. Chem Commun 53(2):424–427
Wu S et al (2015) Colorimetric aptasensor based on enzyme for the detection of Vibrio parahemolyticus. J Agric Food Chem 63(35):7849–7854
Ziegler C, Eychmüller A (2011) Seeded growth synthesis of uniform gold nanoparticles with diameters of 15–300 nm. J Phys Chem C 115(11):4502–4506
Yao S et al (2020) Colorimetric immunoassay for the detection of Staphylococcus aureus by using magnetic carbon dots and sliver nanoclusters as o-phenylenediamine-oxidase mimetics. Food Anal Methods 13(4):833–838
de la Rica R, Velders AH (2011) Supramolecular au nanoparticle assemblies as optical probes for enzyme-linked immunoassays. Small 7(1):66–69
Zhang L et al (2016) Rapid and visual detection of Listeria monocytogenes based on nanoparticle cluster catalyzed signal amplification. Biosens Bioelectron 86:1–7
Hossein-Nejad-Ariani H, Kim T, Kaur K (2018) Peptide-based biosensor utilizing fluorescent gold nanoclusters for detection of Listeria monocytogenes. ACS Appl Nano Mater 1(7):3389–3397
Wei X et al (2018) Multiplexed instrument-free bar-chart SpinChip integrated with nanoparticle-mediated magnetic aptasensors for visual quantitative detection of multiple pathogens. Anal Chem 90(16):9888–9896
Chen R et al (2015) Plasmonic enzyme-linked immunosorbent assay using nanospherical brushes as a catalase container for colorimetric detection of ultralow concentrations of Listeria monocytogenes. ACS Appl Mater Interfaces 7(51):28632–28639
Chen Q et al (2015) A sensitive impedance biosensor based on immunomagnetic separation and urease catalysis for rapid detection of Listeria monocytogenes using an immobilization-free interdigitated array microelectrode. Biosens Bioelectron 74:504–511
Funding
The authors thank the financial support from the National Natural Science Foundation of China (Grant No. 82073603 and 82073557), Jilin Province Science, and Technology Development Plan Item (Grant No. 20200403035SF).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, X., Liu, Y., Shi, X. et al. Colorimetric determination of Listeria monocytogenes using aptamer and urease dual-labeled magnetic nanoparticles and cucurbit[7]uril-mediated supramolecular assembly of gold nanoparticle. Microchim Acta 189, 41 (2022). https://doi.org/10.1007/s00604-021-05130-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-05130-3