Abstract
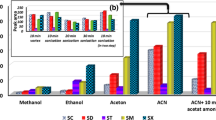
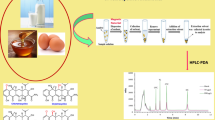
An ionic liquid-based dispersive liquid–liquid microextraction (IL-DLLME) combined with magnetic solid-phase extraction (MSPE) was developed for extraction of quinolones (quinolones) from honey and milk prior to high-performance liquid chromatography (HPLC) analysis. 1-Butyl-3-methylimidazolium hexafluorophosphate was used as the extraction solvent and an effective adsorbent based on chitosan modified magnetic core–shell functionalized multi-walled carbon nanotube (MWCNTs-Fe3O4@SiO2-CS) nanoparticles was used to assist IL to adsorb quinolone residues in honey and milk samples. Extraction conditions were optimized through one-factor-at-a-time and response surface methodology using a Box-Behnken design. Under optimum conditions satisfactory linearity (R2 > 0.999) and high sensitivity (method limits of quantification were 4–8 μg kg−1 or μg L−1 in honey or milk samples) was achieved. The recoveries of quinolones in honey and milk ranged from 81.2 to 109%. Based on this study, the proposed method was employed for the determination of antibiotic residues in honey and milk samples.
Graphical abstract




Similar content being viewed by others
References
Hernández-Arteseros JA, Barbosa J, Compano R, Prat MD (2002) Analysis of quinolone residues in edible animal products. J Chromatogr A 945:1–24. https://doi.org/10.1016/S0021-9673(01)01505-9
Jin Y, Zhang J, Zhao W, Zhang W, Wang L, Zhou J, Li Y (2017) Development and validation of a multiclass method for the quantification of veterinary drug residues in honey and royal jelly by liquid chromatography-tandem mass spectrometry. Food Chem 221:1298–1307. https://doi.org/10.1016/j.foodchem.2016.11.026
Pereira PC (2014) Milk nutritional composition and its role in human health. Nutrition 30:619–627. https://doi.org/10.1016/j.nut.2013.10.011
Cui X, Zhang P, Yang X, Yang M, Zhou W, Zhang S, Gao H, Lu R (2015) β-CD/ATP composite materials for use in dispersive solid-phase extraction to measure (fluoro) quinolone antibiotics in honey samples. Anal Chim Acta 878:131–139. https://doi.org/10.1016/j.aca.2015.03.056
Zayas-Blanco FD, Garcı́a-Falcón MS, Simal-Gándara J (2004) Determination of sulfamethazine in milk by solid phase extraction and liquid chromatographic separation with ultraviolet detection. Food Control 15:375–378. https://doi.org/10.1016/S0956-7135(03)00100-2
Zhao B, Wu H, Liu Y, Tian X, Huo Y, Guan S (2019) Magnetic solid-phase extraction based on g-C3N4/Fe3O4/MoS2 as a magnetic adsorbent for HPLC-UV determination of fluoroquinolones in chicken and eggs. Anal Methods 11:1491–1499. https://doi.org/10.1039/C9AY00208A
Rodríguez-Díaz RC, Fernández-Romero JM, Aguilar-Caballos MP, Gómez-Hens A (2006) Chromatographic determination of flumequine in food samples by post-column derivatisation with terbium(III). Anal Chim Acta 578:220–226. https://doi.org/10.1016/j.aca.2006.06.068
Chung H, Lee J, Chung Y, Lee K (2009) Analysis of sulfonamide and quinolone antibiotic residues in Korean milk using mi-crobial assays and high performance liquid chromatography. Food Chem 113:297–301. https://doi.org/10.1016/j.foodchem.2008.07.021
Tayeb-Cherif K, Peris-Vicente J, Carda-Broch S, Esteve-Romero J (2016) Use of micellar liquid chromatography to analyze oxolinic acid, flumequine, marbofloxacin and enrofloxacin in honey and validation according to the 2002/657/EC decision. Food Chem 202:316–323. https://doi.org/10.1016/j.foodchem.2016.02.007
Liu XM, Feng SY, Zhou P, Chen YQ, Zhang H, Chen W (2013) Simultaneous determination of danofloxacin and flumequine in milk based on fluorescence spectroscopy and chemometrics tools food. Anal Methods 6:1739–1749. https://doi.org/10.1007/s12161-013-9702-9
Jin T, Wu H, Gao NN, Chen XD, Lai HJ, Zheng JF, Du LM (2016) Extraction of quinolones from milk samples using bentonite/magnetite nanoparticles before determination by high-performance liquid chromatography with fluorimetric detection. J Sep Sci 39:545–551. https://doi.org/10.1002/jssc.201500856
Junza A, Dorival-García N, Zafra-Gómez A, Barrón D, Ballesteros O, Barbosa J, Navalón A (2014) Multiclass method for the determination of quinolones and β-lactams, in raw cow milk using dispersive liquid-liquid microextraction and ultra high performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1356:10–22. https://doi.org/10.1016/j.chroma.2014.06.034
Parrilla Vázquez MM, Parrilla Vázquez P, Martínez Galera M, Gil García MD, Uclés A (2013) Ultrasound-assisted ionic liquid dispersive liquid-liquid microextraction coupled with liquid chromatography-quadrupole-linear ion trap-mass spectrometry for simultaneous analysis of pharmaceuticals in wastewaters. J Chromatogr A 1219:19–26. https://doi.org/10.1016/j.chroma.2013.03.066
Xu S, Jiang C, Lin YX, Jia L (2012) Magnetic nanoparticles modified with polydimethylsiloxane and multi-walled carbon nanotubes for solid-phase extraction of fluoroquinolones. Microchem Acta 179:257–264. https://doi.org/10.1007/s00604-012-0894-2
Xu JJ, An M, Yang R, Tan Z, Hao J, Cao J, Peng LQ, Cao W (2016) Determination of tetracycline antibiotic residues in honey and milk by miniaturized solid phase extraction using chitosan-modified graphitized multiwalled carbon nanotubes. J Agr Food Chem 64:2647–2654. https://doi.org/10.1021/acs.jafc.6b00748
Yuan XC, Li XH, Guo P, Xiong ZL, Zhao LS (2018) Simultaneous enantiomeric analysis of chiral non-steroidal anti-inflammatory drugs in water, river sediment and sludge by chiral liquid chromatography-tandem mass spectrometry. Anal Methods 10:4404–4413. https://doi.org/10.1039/C8AY01417E
Zarnegar Z, Safari J (2015) The novel synthesis of magnetically chitosan/carbon nanotube composites and their catalytic applications. Int J Biol Macromol 75:21–31. https://doi.org/10.1016/j.ijbiomac.2015.01.013
Shi ZG, Lee HK (2010) Dispersive liquid-liquid microextraction coupled with dispersive µ-solid-phase extraction for the fast determination of polycyclic aromatic hydrocarbons in environmental water samples. Anal Chem 82:1540–1545. https://doi.org/10.1021/ac9023632
Zhou J, Xu J, Cong J, Cai Z, Zhang J, Wang J, Ren Y (2018) Optimization for quick, easy, cheap, effective, rugged and safe extraction of mycotoxins and veterinary drugs by response surface methodology for application to egg and milk. J Chromatogr A 1532:20–29. https://doi.org/10.1016/j.chroma.2017.11.050
Gao F, Hu Y, Ye X, Li J, Chen Z, Fan G (2013) Optimal extraction and fingerprint analysis of Cnidii fructus by accelerated solvent extraction and high performance liquid chromatographic analysis with photodiode array and mass spectrometry detections. Food Chem 141:1962–1971. https://doi.org/10.1016/j.foodchem.2013.05.013
Tang W, Zeng G, Gong J, Liu Y, Wang X, Liu Y, Liu Z, Chen L, Zhang X, Tu D (2012) Simultaneous adsorption of atrazine and Cu(II) from wastewater by magnetic multi-walled carbon nanotube. Chem Eng J 211–212:470–478. https://doi.org/10.1016/j.cej.2012.09.102
Tiwari A, Dhakate SR (2009) Chitosan-SiO2-multiwall carbon nanotubes nanocomposite: a novel matrix for the immobilization of creatine amidinohydrolase. Int J Biol Macromol 44:408–412. https://doi.org/10.1016/j.ijbiomac.2009.03.002
Sadeghi S, Olieaei S (2019) Nanostructured polyaniline based pipette tip solid phase extraction coupled with high-performance liquid chromatography for the selective determination of trace levels of three sulfonamides in honey and milk samples with the aid of experimental design methodology. Microchem J 146:974–985. https://doi.org/10.1016/j.microc.2019.02.020
Kong L, Lu X, Zhang W (2008) Facile synthesis of multifunctional multiwalled carbon nanotubes/Fe3O4 nanoparticles/polyaniline composite nanotubes. J Solid State Chem 181:628–636. https://doi.org/10.1016/j.jssc.2008.01.006
Zhou LX, Pan SD, Chen XH, Zhao YG, Zou BB, Jin MC (2014) Kinetics and thermodynamics studies of pentachlorophenol adsorption on covalently functionalized Fe3O4@SiO2-MWCNTs core-shell magnetic microspheres. Chem Eng J 257:10–19. https://doi.org/10.1016/j.cej.2014.07.060
Ning J, Zhang J, Pan Y, Guo J (2003) Fabrication and thermal property of carbon nanotube/SiO2 composites. J Mater Sci 22:1019–1021. https://doi.org/10.1023/A:1024745526978
Lu N, He X, Wang T, Liu S, Hou X (2018) Magnetic solid-phase extraction using MIL-101(Cr)-based composite combined with dispersive liquid-liquid microextraction based on solidification of a floating organic droplet for the determination of pyrethroids in environmental water and tea samples. Microchem J 137:449–455. https://doi.org/10.1016/j.microc.2017.12.009
Wang Y, Zhang J, Huang X, Yuan D (2014) Preparation of stir cake sorptive extraction based on polymeric ionic liquid for the enrichment of benzimidazole anthelmintics in water, honey and milk samples. Anal Chim Acta 840:33–41. https://doi.org/10.1016/j.aca.2014.06.039
Di X, Wang X, Liu Y, Guo X, Di X (2019) Dissolvable layered double hydroxide as a sorbent in dispersive micro-solid phase extraction for the determination of acidic quinolones in honey by HPLC. J Sep Sci 42:2255–2262. https://doi.org/10.1002/jssc.201801009
Bohm DA, Stachel CS, Gowik P (2012) Validation of a multi-residue method for the determination of several antibiotic groups in honey by LC-MS/MS. Anal Bioanal Chem 403:2943–2953. https://doi.org/10.1007/s00216-012-5868-z
Ibarra IS, Rodriguez JA, Páez-Hernández ME, Santos EM, Miranda JM (2012) Determination of quinolones in milk samples using a combination of magnetic solid-phase extraction and capillary electrophoresis. Electrophoresis 33:2041–2048. https://doi.org/10.1002/elps.201100559
Chung HH, Lee JB, Chung YH, Lee KG (2009) Analysis of sulfonamide and quinolone antibiotic residues in Korean milk using microbial assays and high performance liquid chromatography. Food Chem 113:297–301. https://doi.org/10.1016/j.foodchem.2008.07.021
Gao S, Jin H, You J, Ding Y, Zhang N, Wang Y, Ren R, Zhang R, Zhang H (2011) Ionic liquid-based homogeneous liquid-liquid microextraction for the determination of antibiotics in milk by high-performance liquid chromatography. J Chromatogr A 1218:7254–7263. https://doi.org/10.1016/j.chroma.2011.08.063
Wang H, Liu Y, Wei S, Yao S, Zhang J, Huang H (2016) Selective extraction and determination of fluoroquinolones in bovine milk samples with montmorillonite magnetic molecularly imprinted polymers and capillary electrophoresis. Anal Bioanal Chem 408:589–598. https://doi.org/10.1007/s00216-015-9140-1
Zheng H, Mo J, Zhang Y, Gao Q, Ding J, Yu QW, Feng Y (2014) Facile synthesis of magnetic molecularly imprinted polymers and its application in magnetic solid phase extraction for fluoroquinolones in milk samples. J Chromatogr A 1329:17–23. https://doi.org/10.1016/j.chroma.2013.12.083
Acknowledgements
This work was supported by Hainan Provincial Philosophy and Social Science Planning Project (HNSK(YB)20-64), the Middle-aged Backbone Personnel Training Program of Shenyang Pharmaceutical University (ZQN2016011), Scientific Research Fund of Liaoning Provincial Education Department (2020LZD02), Inter-school Cooperation Project of General Undergraduate Universities in Liaoning Province (2020-181), and Project of Shenyang Key Laboratory of Functional Drug Carrier Materials (19-110-4-08).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hong, J., Liu, X., Yang, X. et al. Ionic liquid-based dispersive liquid–liquid microextraction followed by magnetic solid-phase extraction for determination of quinolones. Microchim Acta 189, 8 (2022). https://doi.org/10.1007/s00604-021-05077-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-05077-5