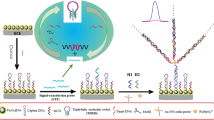
Abstract
A three-dimensional (3D) micromolecule walking nanomachine propelled by strand displacement was developed to establish a novel switching electrochemiluminescence (ECL) biosensor for ultrasensitive detection of adenosine triphosphate (ATP). Generally, walking nanomachines reported previously were limited to DNA walkers, while the proposed 3D walking nanomachine focused on the micromolecule walker. Firstly, TiO2 and silver nanoparticles (Ag NPs) functionalized N-(4-aminobutyl)-N-(ethylisoluminol) (Ag-ABEI) were deposited onto the electrode surface to offer an enhanced ECL signal, resulting from the double catalytic effect of TiO2 and Ag NPs for H2O2. Following, dopamine (DA)-labeled DNA duplex probes (S1/S2-DA) immobilized onto the modified electrode cut down the original ECL signal due to the quenching of DA toward ABEI (signal-off). Target ATP walker moved along the 3D DNA track, simultaneously releasing numerous DNA3, which was applied to displace S2-DA, resulting in the quenched ECL intensity recovery (signal-on). As a result, the biosensor showed a low limit of detection down to 0.5 nM (S/N = 3) and was successfully employed to determine ATP in human serum samples. Thus, the established biosensing strategy holds great potential for biochemical studies and clinical diagnosis.
Graphical abstract







Similar content being viewed by others
References
Chen JY, Liu YC, Ji XH, He ZK (2016) Target-protecting dumbbell molecular probe against exonucleases digestion for sensitive detection of ATP and streptavidin. Biosens Bioelectron 83:221–228
Gourine AV, Llaudet E, Dale N, Spyer KM (2005) ATP is a mediator of chemosensory transduction in the central nervous system. Nature 436:108–111
Fan YY, Mou ZL, Wang M, Li J, Zhang J, Dang FQ, Zhang ZQ (2018) Chimeric aptamers-based and MoS2 nanosheet-enhanced label-free fluorescence polarization strategy for adenosine triphosphate detection. Anal Chem 90:13708–13713
Gorman MW, Feigl EO, Buffington CW (2007) Human plasma ATP concentration. Clin Chem 53:318–325
Zhang XY, Song CX, Yang K, Hong WW, Lu Y, Yu P, Mao LQ (2018) Photo-induced regeneration of an aptamer-based electrochemical sensor for sensitively detecting adenosine triphosphate. Anal Chem 90:4968–4971
Lu SS, Wang S, Chen CX, Sun J, Yang XR (2018) Enzyme-free aptamer/AuNPs-based fluorometric and colorimetric dual-mode detection for ATP. Sens Actuators B-Chem 265:67–74
Huang YF, Chang HT (2007) Analysis of adenosine triphosphate and glutathione through gold nanoparticles assisted laser desorption/ionization mass spectrometry. Anal Chem 79:4852–4859
Xue XF, Wang F, Zhou JH, Chen F, Li Y, Zhao J (2009) Online cleanup of accelerated solvent extractions for determination of adenosine 5’-triphosphate (ATP), adenosine 5’-diphosphate (ADP), and adenosine 5’-monophosphate (AMP) in royal jelly using high-performance liquid chromatography. J Agric Food Chem 57:4500–4505
Huang LX, Guo Q, Chen Y, Verwilst P, Son SB, Wu JB, Cao QY, Kim JS (2019) Nanomolar detection of adenosine triphosphate (ATP) using a nanostructured fluorescent chemosensing ensemble. Chem Commun 55:14135–14138
Li X, Yang JM, Xie JQ, Jiang BY, Yuan R, Xiang Y (2018) Cascaded signal amplification via target-triggered formation of aptazyme for sensitive electrochemical detection of ATP. Biosens Bioelectron 102:296–300
Liu YT, Lei JP, Huang Y, Ju HX (2014) “off-on” electrochemiluminescence system for sensitive detection of ATP via target-induced structure switching. Anal Chem 86:8735–8741
Ma W, Xu LG, de Moura AF, Wu XL, Kuang H, Xu CL, Kotov NA (2017) Chiral inorganic nanostructures. Chem Rev 117:8041–8093
Karimi A, Husain SW, Hosseini M, Azar PA, Ganjali MR (2020) A sensitive signal-on electrochemiluminescence sensor based on a nanocomposite of polypyrrole-Gd2O3 for the determination of L-cysteine in biological fluids. Microchim Acta 187:398–407
Lu LX, Yuan W, Xiong Q, Wang MH, Liu YJ, Cao M, Xiong XH (2021) One-step grain pretreatment for ochratoxin A detection based on bipolar electrode- electrochemiluminescence biosensor. Anal Chim Acta 1141:83–90
Azam NFN, Mohd-Naim NF, Kurup CP, Ahmed MU (2020) Electrochemiluminescence immunosensor for tropomyosin using carbon nanohorns/Nafion/Fe3O4@Pd screen-printed electrodes. Microchim Acta 187:456–465
Lu LP, Liu C, Miao WJ, Wang XY, Guo GS (2020) Ultrasensitive detection of miRNA based on efficient immobilization of probe and electrochemiluminescent quenching of Ru(bpy)32+ by methylene blue. Anal Chim Acta 1093:52–60
Ma C, Cao Y, Gou XD, Zhu JJ (2020) Recent progress in electrochemiluminescence sensing and imaging. Anal Chem 92:431–454
Li SK, Liu ZT, Li JY, Chen AY, Chai YQ, Yuan R, Zhuo Y (2018) Enzyme-free target recycling and double-output amplification system for electrochemiluminescent assay of mucin1 with MoS2 nanoflowers as co-reaction accelerator. ACS Appl Mater Interfaces 10:14483–14490
Yang F, Jiang XY, Zhong X, Wei SP, Yuan R (2018) Highly sensitive electrochemiluminescence detection of mucin1 based on V2O5 nanospheres as peroxidase mimetics to catalyze H2O2 for signal amplification. Sens Actuators B-Chem 265:126–133
Xu LH, Li JJ, Zeng HB, Zhang XJ, Cosnier S, Marks RS, Shan D (2019) ATMP-induced three-dimensional conductive polymer hydrogel scaffold for a novel enhanced solid-state electrochemiluminescence biosensor. Biosens Bioelectron 143:111601–111608
Li J, Jiang D, Shan X, Wang W, Chen Z (2020) An “off-on” electrochemiluminescence aptasensor for microcystin-LR assay based on the resonance energy transfer from PTCA/NH2-MIL-125(Ti) to gold nanoparticles. Microchim Acta 187:474–483
Wang HQ, Ma ZF (2019) “off-on” signal amplification strategy amperometric immunosensor for ultrasensitive detection of tumour marker. Biosens Bioelectron 132:265–270
Gao XS, Li HK, Zhao Y, Jie GF (2019) Triple-helix molecular switch-based versatile “off-on” electrochemiluminescence and fluorescence biosensing platform for ultrasensitive detection of lipopolysaccharide by multiple-amplification strategy. Biosens Bioelectron 143:111602–111610
Qu XM, Zhu D, Yao GB, Su S, Chao J, Liu HJ, Zuo XL, Wang LH, Shi JY, Wang LH, Huang W, Pei H, Fan CH (2017) An exonuclease III-powered, on-particle stochastic DNA walker. Angew Chem Int Ed 56:1855–1858
Lu HW, Tang HL, Yi XY, Wang JX (2020) Three-dimensional DNA nanomachine combined with toehold-mediated strand displacement reaction for sensitive electrochemical detection of MiRNA. Langmuir 36:10708–10714
Qi XJ, Lu CH, Liu XQ, Shimron S, Yang HH, Willner I (2013) Autonomous control of interfacial electron transfer and the activation of DNA machines by an oscillatory pH system. Nano Lett 13:4920–4924
Thubagere AJ, Li W, Johnson RF, Chen ZB, Doroudi S, Lee YL, Izatt G, Wittman S, Srinivas N, Woods D, Winfree E, Qian LL (2017) A cargo-sorting DNA robot. Science 357:6356–6358
Li DD, Xu YX, Fan L, Shen B, Ding XJ, Yuan R, Li XM, Chen WX (2020) Target-driven rolling walker based electrochemical biosensor for ultrasensitive detection of circulating tumor DNA using doxorubicin@tetrahedron-Au tags. Biosens Bioelectron 148:111826–111833
Xiao MS, Zou K, Li L, Wang LH, Tian Y, Fan CH, Pei H (2019) Stochastic DNA walkers in droplets for super-multiplexed bacterial phenotype detection. Angew Chem Int Ed 58:15448–15454
Xu MD, Zhuang JY, Jiang XY, Liu XZ, Tang DP (2019) A three-dimensional DNA walker amplified FRET sensor for detection of telomerase activity based on the MnO2 nanosheet-upconversion nanoparticle sensing platform. Chem Commun 55:9857–9860
Fakhari F, Rokita SE (2014) A walk along DNA using bipedal migration of a dynamic and covalent crosslinker. Nat Commun 5:5591–5601
Jung C, Allen PB, Ellington AD (2017) A simple, cleated DNA walker that hangs on to surfaces. ACS Nano 11:8047–8054
Wang L, Liu PF, Liu ZJ, Zhao KR, Ye SY, Liang GX, Zhu JJ (2020) Simple tripedal DNA walker prepared by target-triggered catalytic hairpin assembly for ultrasensitive electrochemiluminescence detection of microRNA. ACS Sens 5:3584–3590
Yang XL, Tang YN, Mason SD, Chen JB, Li F (2016) Enzyme-powered three-dimensional DNA nanomachine for DNA walking, payload release, and biosensing. ACS Nano 10:2324–2330
Wang CQ, Liu R, Hu JY, Lv Y (2019) Ratiometric DNA walking machine for accurate and amplified bioassay. Chem- Eur J 25:12270–12274
Murphy CJ, Chang HH, Falagan-Lotsch P, Gole MT, Hofmann DM, Hoang KNL, McClain SM, Meyer SM, Turner JG, Unnikrishnan M, Wu M, Zhang X, Zhang YZ (2019) Virus-sized gold nanorods: plasmonic particles for biology. Acc Chem Res 52:2124–2135
Li Q, Liang XH, Mu XM, Tan L, Lu JN, Hu K, Zhao SL, Tian JN (2020) Ratiometric fluorescent 3D DNA walker and catalyzed hairpin assembly for determination of microRNA. Microchim Acta 187:365–374
Funding
This work was funded by financial support from the National Natural Science Foundation of China (82002257, 81702083), the Natural Science Foundation Project of Chongqing (cstc2020jcyj-msxmX0190), the Science and Technology Plan Project of Yuzhong District of Chongqing (20170128), and Hospital-special Fund of Chengdu University of TCM (YYZX20180030).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOC 1172 kb)
Rights and permissions
About this article
Cite this article
Li, L., Zhou, Z., Li, X. et al. An “off-on” electrochemiluminescence biosensor coupled with strand displacement-powered 3D micromolecule walking nanomachine for ultrasensitive detection of adenosine triphosphate. Microchim Acta 188, 237 (2021). https://doi.org/10.1007/s00604-021-04895-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04895-x