Abstract
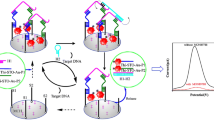
A novel electrochemiluminescence (ECL) biosensor was fabricated for miRNA-162a detection by using silver nanoclusters/molybdenum disulfide (AgNCs@MoS2) as an ECL material, peroxodisulfate (S2O82−) as a co-reactant, and semicarbazide (Sem) as a co-reaction accelerator. Firstly, hairpin probe Ha modified on AgNCs@MoS2/GCE was unfolded based on its hybridization with target microRNA. Then, the unfolded Ha can further be hybridized with another hairpin DNA of Hb on (AuNPs-semicarbazide)@Cu-MOF, resulting in the release of target microRNA, which further causes a cyclic hybridization. This creates more (AuNPs-semicarbazide)@Cu-MOF on the electrode surface, achieving cyclic hybridization signal amplification. Strikingly, due to the presence of Sem, accelerating the reduction of S2O82− and resulting in the generation of more oxidant intermediates of SO42−, the amount of excited states of Agincreases to further amplify the ECL signal. The biosensor exhibited high sensitivity with a low LOD of 1.067 fM, indicating that the introduction of co-reaction accelerators can provide an effective method for signal amplification. The applicability of this method was assessed by investigating the effect of Pb(II) ion on miRNA-162a expression level in maize seedling leaves.
Graphical abstract

A novel electrochemiluminescence biosensor was fabricated for miRNA-162a detection by using silver nanoclusters/molybdenum disulfide as an ECL material, peroxodisulfate as a co-reactant, and semicarbazide as a co-reaction accelerator.





Similar content being viewed by others
References
Bose D, Jayaraj GG, Kumar S, Maiti S (2013) A molecular-beacon-based screen for small molecule inhibitors of miRNA maturation. ACS Chem Biol 8(5):930–938. https://doi.org/10.1021/cb300650y
Jia H, Li Z, Liu C, Cheng Y (2010) Ultrasensitive detection of microRNAs by exponential isothermal amplification. Angew Chem Int Edit 49(32):5498–5501. https://doi.org/10.1002/anie.201001375
Yang GD, Li YY, Wu BJ, Zhang KY, Gao L, Zheng CC (2019) MicroRNAs transcriptionally regulate promoter activity in Arabidopsis thaliana. J Integr Plant Biol 61(11):1128–1133. https://doi.org/10.1111/jipb.12775
Su YH, Liu YB, Zhou C, Li XM, Zhang XS (2016) The microRNA167 controls somatic embryogenesis in Arabidopsis through regulating its target genes ARF6 and ARF8. Plant Cell Tiss Org 124(2):405–417. https://doi.org/10.1007/s11240-015-0903-3
Wu L, Liu DF, Wu JJ, Zhang RZ, Qin ZR, Liu DM, Li AL, Fu DL, Zhai WX, Mao L (2013) Regulation of FLOWERING LOCUS T by a microRNA in Brachypodium distachyon. Plant Cell 25(11):4363–4377. https://doi.org/10.1105/tpc.113.118620
Zhang X, Li K, Xing R, Liu S, Chen X, Yang H, Li P (2018) MiRNA and mRNA expression profiles reveal insight into chitosan-mediated regulation of plant growth. J Agr Food Chem 66(15):3810–3822. https://doi.org/10.1021/acs.jafc.7b06081
Ouyang T, Liu Z, Han Z, Ge Q (2019) MicroRNA detection specificity: recent advances and future perspective. Anal Chem 91(5):3179–3186. https://doi.org/10.1021/acs.analchem.8b05909
Dong H, Lei J, Ding L, Wen Y, Ju H, Zhang X (2013) MicroRNA: function, detection, and bioanalysis. Chem Rev 113(8):6207–6233. https://doi.org/10.1021/cr300362f
Hunt EA, Broyles D, Head T, Deo SK (2015) MicroRNA detection: current technology and research strategies. Annu Rev Anal Chem 8(1):217–237. https://doi.org/10.1146/annurev-anchem-071114-040343
Xu J, Yan C, Wang X, Yao B, Lu J, Liu G, Chen W (2019) Ingenious design of DNA concatamers and G-quadruplex wires assisted assembly of multibranched DNA nanoarchitectures for ultrasensitive biosensing of miRNA. Anal Chem 91(15):9747–9753. https://doi.org/10.1021/acs.analchem.9b01353
Akbarnia A, Zare HR (2018) A voltammetric assay for microRNA-25 based on the use of amino-functionalized graphene quantum dots and ss- and ds-DNAs as gene probes. Microchim. Acta 185(11):503. https://doi.org/10.1007/s00604-018-3037-6
Lee J, Kim Y-k, Lee S, Yoon S, Kim W-k (2019) Graphene oxide-based NET strategy for enhanced colorimetric sensing of miRNA. Sensor Actuat B-Chem 282:861–867. https://doi.org/10.1016/j.snb.2018.11.149
Chen X, Xu K, Li J, Yang M, Li X, Chen Q, Lu C, Yang H (2020) Switch-conversional ratiometric fluorescence biosensor for miRNA detection. Biosens Bioelectron 155:112104. https://doi.org/10.1016/j.bios.2020.112104
Cao X, Zhang K, Yan W, Xia Z, He S, Xu X, Ye Y, Wei Z, Liu S (2020) Calcium ion assisted fluorescence determination of microRNA-167 using carbon dots–labeled probe DNA and polydopamine-coated Fe3O4 nanoparticles. Microchim Acta 187(4):212. https://doi.org/10.1007/s00604-020-4209-8
Wu H, Wang H, Liu Y, Wu J, Zou P (2019) Fluorometric determination of microRNA by using target-triggered cascade signal amplification and DNA-templated silver nanoclusters. Microchim Acta 186(10):669. https://doi.org/10.1007/s00604-019-3789-7
Wang Y, Shi H, Cui K, Zhang L, Ge S, Yan M, Yu J (2018) Hierarchical hematite/TiO2 nanorod arrays coupled with responsive mesoporous silica nanomaterial for highly sensitive photoelectrochemical sensing. Biosens Bioelectron 117:515–521. https://doi.org/10.1016/j.bios.2018.06.030
Wang M, Yin H, Zhou Y, Han J, He T, Cui L, Ai S (2018) Photoelectrochemical biosensor for microRNA detection based on multiple amplification strategies. Microchim Acta 185(5):257. https://doi.org/10.1007/s00604-018-2808-4
Liu C, Zhao L, Liang D, Zhang X, Song W (2019) An CuInS2 photocathode for the sensitive photoelectrochemical determination of microRNA-21 based on DNA–protein interaction and exonuclease III assisted target recycling amplification. Microchim Acta 186(11):692. https://doi.org/10.1007/s00604-019-3804-z
Liu G, Hong J, Ma K, Wan Y, Zhang X, Huang Y, Kang K, Yang M, Chen J, Deng S (2020) Porphyrin Trio−Pendant fullerene guest as an in situ universal probe of high ECL efficiency for sensitive miRNA detection. Biosens Bioelectron 150:111963. https://doi.org/10.1016/j.bios.2019.111963
Li Z, Lin Z, Wu X, Chen H, Chai Y, Yuan R (2017) Highly efficient electrochemiluminescence resonance energy transfer system in one nanostructure: its application for ultrasensitive detection of microRNA in cancer cells. Anal Chem 89(11):6029–6035. https://doi.org/10.1021/acs.analchem.7b00616
Zhang R, Chen A, Yu Y, Chai Y, Zhuo Y, Yuan R (2018) Electrochemiluminescent carbon dot-based determination of microRNA-21 by using a hemin/G-wire supramolecular nanostructure as co-reaction accelerator. Microchim Acta 185(9):432. https://doi.org/10.1007/s00604-018-2959-3
Zhong X, Li X, Zhuo Y, Chai Y, Yuan R (2020) Synthesizing anode electrochemiluminescent self-catalyzed carbon dots-based nanocomposites and its application in sensitive ECL biosensor for microRNA detection. Sensor Actuat B-Chem 305:127490. https://doi.org/10.1016/j.snb.2019.127490
Shao H, Lu J, Zhang Q, Hu Y, Wang S, Guo Z (2018) Ruthenium-based metal organic framework (Ru-MOF)-derived novel Faraday-cage electrochemiluminescence biosensor for ultrasensitive detection of miRNA-141. Sensor Actuat B-Chem 268:39–46. https://doi.org/10.1016/j.snb.2018.04.088
Piñeiro Y, Buceta D, Calvo J, Huseyinova S, Cuerva M, Pérez Á, Domínguez B, López-Quintela MA (2015) Large stability and high catalytic activities of sub-nm metal (0) clusters: implications into the nucleation and growth theory. J Colloid Interf Sci 449:279–285. https://doi.org/10.1016/j.jcis.2015.01.001
Watanabe K, Welling TAJ, Sadighikia S, Ishii H, Imhof A, van Huis MA, van Blaaderen A, Nagao D (2020) Compartmentalization of gold nanoparticle clusters in hollow silica spheres and their assembly induced by an external electric field. J Colloid Interf Sci 566:202–210. https://doi.org/10.1016/j.jcis.2020.01.094
Mandal S, Wang J, Winans RE, Jensen L, Sen A (2013) Quantum size effects in the optical properties of ligand stabilized aluminum nanoclusters. J Phys Chem C 117(13):6741–6746. https://doi.org/10.1021/jp310514z
Chen A, Ma S, Zhuo Y, Chai Y, Yuan R (2016) In situ electrochemical generation of electrochemiluminescent silver naonoclusters on target-cycling synchronized rolling circle amplification platform for microRNA detection. Anal Chem 88(6):3203–3210. https://doi.org/10.1021/acs.analchem.5b04578
Yang H-Y, Wang H-J, Xiong C-Y, Chai Y-Q, Yuan R (2017) Intramolecular self-enhanced nanochains functionalized by an electrochemiluminescence reagent and its immunosensing application for the detection of urinary β2-microglobulin. ACS Appl Mater Inter 9(41):36239–36246. https://doi.org/10.1021/acsami.7b12011
Irkham WT, Fiorani A, Valenti G, Paolucci F, Einaga Y (2016) Co-reactant-on-demand ECL: Electrogenerated chemiluminescence by the in situ production of S2O82− at boron-doped diamond electrodes. J Am Chem Soc 138(48):15636–15641. https://doi.org/10.1021/jacs.6b09020
Lei Y-M, Wen R-X, Zhou J, Chai Y-Q, Yuan R, Zhuo Y (2018) Silver ions as novel coreaction accelerator for remarkably enhanced electrochemiluminescence in a PTCA–S2O82− system and its application in an ultrasensitive assay for mercury ions. Anal Chem 90(11):6851–6858. https://doi.org/10.1021/acs.analchem.8b01018
Ma M-N, Zhuo Y, Yuan R, Chai Y-Q (2015) New signal amplification strategy using semicarbazide as co-reaction accelerator for highly sensitive electrochemiluminescent aptasensor construction. Anal Chem 87(22):11389–11397. https://doi.org/10.1021/acs.analchem.5b02848
Sun Y, Wu X, Zhang K, Ren Q, Xie R (2018) Electrochemiluminescent quaternary Cu-Zn-In-S nanocrystals as a sensing platform: enzyme-free and sensitive detection of the FLT3 gene based on triple signal amplification. Biosens Bioelectron 100:445–452. https://doi.org/10.1016/j.bios.2017.09.026
Dong X, Zhao G, Li X, Miao J, Fang J, Wei Q, Cao W (2019) Electrochemiluminescence immunoassay for the N-terminal pro-B-type natriuretic peptide based on resonance energy transfer between a self-enhanced luminophore composed of silver nanocubes on gold nanoparticles and a metal-organic framework of type MIL-125. Microchim Acta 186(12):811. https://doi.org/10.1007/s00604-019-3969-5
Feng L, Wu L, Xing F, Hu L, Ren J, Qu X (2017) Novel electrochemiluminescence of silver nanoclusters fabricated on triplex DNA scaffolds for label-free detection of biothiols. Biosens Bioelectron 98:378–385. https://doi.org/10.1016/j.bios.2017.07.016
Chen Y, Zhou Y, Yin H, Li F, Li H, Guo R, Han Y, Ai S (2020) Photoelectrochemical biosensor for histone acetyltransferase detection based on ZnO quantum dots inhibited photoactivity of BiOI nanoflower. Sensor Actuat B-Chem 307:127633. https://doi.org/10.1016/j.snb.2019.127633
Bhardwaj SK, Bhardwaj N, Mohanta GC, Kumar P, Sharma AL, Kim K-H, Deep A (2015) Immunosensing of atrazine with antibody-functionalized Cu-MOF conducting thin films. ACS Appl Mater Inter 7(47):26124–26130. https://doi.org/10.1021/acsami.5b07692
Wang H, Jian Y, Kong Q, Liu H, Lan F, Liang L, Ge S, Yu J (2018) Ultrasensitive electrochemical paper-based biosensor for microRNA via strand displacement reaction and metal-organic frameworks. Sensor Actuat B-Chem 257:561–569. https://doi.org/10.1016/j.snb.2017.10.188
Miao P, Wang B, Chen X, Li X, Tang Y (2015) Tetrahedral DNA nanostructure-based microRNA biosensor coupled with catalytic recycling of the analyte. ACS Appl Mater Inter 7(11):6238–6243. https://doi.org/10.1021/acsami.5b01508
Lv W, Zhao J, Situ B, Li B, Ma W, Liu J, Wu Z, Wang W, Yan X, Zheng L (2016) A target-triggered dual amplification strategy for sensitive detection of microRNA. Biosens Bioelectron 83:250–255. https://doi.org/10.1016/j.bios.2016.04.053
Wang B, Dong Y-X, Wang Y-L, Cao J-T, Ma S-H, Liu Y-M (2018) Quenching effect of exciton energy transfer from CdS:Mn to Au nanoparticles: a highly efficient photoelectrochemical strategy for microRNA-21 detection. Sens. Actuators B 254:159–165. https://doi.org/10.1016/j.snb.2017.07.078
Zhu H-Y, Ding S-N (2019) Dual-signal-amplified electrochemiluminescence biosensor for microRNA detection by coupling cyclic enzyme with CdTe QDs aggregate as luminophor. Biosens Bioelectron 134:109–116. https://doi.org/10.1016/j.bios.2019.04.005
Lu L, Liu C, Kang T, Wang X, Guo G, Miao W (2018) In situ enhanced electrochemiluminescence based on co-reactant self-generated for sensitive detection of microRNA. Sensor Actuat B-Chem 255:35–41. https://doi.org/10.1016/j.snb.2017.08.029
Fu R, Zhang M, Zhao Y, He X, Ding C, Wang S, Feng Y, Song X, Li P, Wang B (2017) Identification of salt tolerance-related micrornas and their targets in maize (Zea mays L.) using high-throughput sequencing and degradome analysis. Front Plant Sci 8:864
Kong X, Zhang M, Xu X, Li X, Li C, Ding Z (2014) System analysis of microRNAs in the development and aluminium stress responses of the maize root system. Plant Biotechnol J 12(8):1108–1121. https://doi.org/10.1111/pbi.12218
Wu Y, Guo J, Cai Y, Gong X, Xiong X, Qi W, Pang Q, Wang X, Wang Y (2016) Genome-wide identification and characterization of Eutrema salsugineum microRNAs for salt tolerance. Physiol Plantarum 157(4):453–468. https://doi.org/10.1111/ppl.12419
Acknowledgments
This work was supported by the Natural Science Foundation of Shandong Province of China (No. ZR2018MB028), Science and Technology Support Plan for Youth Innovation of Colleges and Universities of Shandong (2019KJC019), and National Natural Science Foundation of China (Nos. 21775090 and 41807484).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 506 kb)
Rights and permissions
About this article
Cite this article
Li, F., Wang, M., Zhou, Y. et al. Electrochemiluminescence biosensor for microRNA determination based on AgNCs@MoS2 composite with (AuNPs-Semicarbazide)@Cu-MOF as coreaction accelerator. Microchim Acta 188, 68 (2021). https://doi.org/10.1007/s00604-020-04678-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04678-w