Abstract
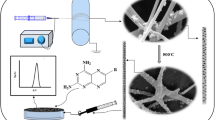
An electrochemical sensor is described for highly sensitive and selective determination of anticancer drug irinitecan (IRT). Gold nanoparticles anchored graphitized carbon nanofibers (Au@GCNFs) was prepared. Au@GCNFs was characterized by X-ray diffraction, scanning electron microscopy, transmission electron microscopy, and energy-dispersive X-ray. The combination of high catalytic activity of the nanocomposite Au@GCNFs and the good conductivity ionic liquid [BMIM]PF6 (IL) resulted in a modified paste electrode (IL/Au@GCNFs-PE). The IL/Au@GCNFs-PE exhibits excellent electrocatalytic activity for selective determination of IRT in the presence of physiological electroactive species, such as ascorbic acid (AA), dopamine (DA), uric acid (UA), and caffeine (CAF) mixture, typically at working potential of 0.88 V vs. Ag/AgCl. The linear response ranges 4.0 nM–1.79 μM and 4.5 nM–1.57 μM with limits of detection of 1.55 nM and 1.70 nM were calculated for IRT in the absence and presence of the quaternary mixture, respectively. The sensor is reproducible and stable over four weeks, and interference by biologically essential compounds is negligible. The method was applied to the determination of IRT in pharmaceutical formulations, in spiked blood serum and urine, and in clinical patient blood. The recovery values ranged from 96.0 to 104.2%.

Graphical abstract
The combination of high catalytic activity of the new nanocomposite AuNPs@GCNFs with the good conductivity ionic liquid (IL) resulted to a modified paste electrode (IL/Au@GCNFs-PE). The novel sensor was successfully applied for the sensitive and selective detection of IRT in biological samples in the presence of quaternary ascorbic acid (AA), dopamine (DA), uric acid (UA), and caffeine (CAF) mixture.






Similar content being viewed by others
References
Sukanya R, Sakthivel M, Chen SM, Chen TW (2018) A new type of terbium diselenide nano octagon integrated oxidized carbon nanofiber: an efficient electrode material for electrochemical detection of morin in the food sample. Sensors Actuators B 269:354–367
Huang J, Liu Y, You T (2010) Carbon nanofiber based electrochemical biosensors: a review. Anal Methods 2:202–211
Mitchell RR, Gallant BM, Thompsona CV, Horn CV (2011) All-carbon-nanofiber electrodes for high-energy rechargeable Li–O2 batteries. Energy Environ Sci 4:2952–2958
Tong Y, Li Z, Lu X, Yang L, Sun W, Nie G, Wang Z, Wang C (2013) Electrochemical determination of dopamine based on electrospun CeO2/Au composite nanofibers. Electrochim Acta 95:12–17
Maleki N, Safavi A, Tajabadi F (2006) High-performance carbon composite electrode based on an ionic liquid as a binder. Anal Chem 78:3820–3826
Liu HT, He P, Li ZY, Sun CY, Shi LH, Liu Y, Zhu GY, Li JH (2005) An ionic iquid-type carbon paste electrode and its polyoxometalate-modified properties. Electrochem Commun 7:1357–1363
Li Y, Zhai X, Liu X, Wang L, Liu H, Wang H (2016) Electrochemical determination of bisphenol A at ordered mesoporous carbon modified nano-carbon ionic liquid paste electrode. Talanta 148:362–369
Mohammadi N, Najafi M, Adeh NB (2017) Highly defective mesoporous carbon-ionic liquid paste electrode as sensitive voltammetric sensor for determination of chlorogenic acid in herbal extracts. Sensors Actuators B Chem 243:838–846
Ping J, Ru S, Fan K, Wu J, Ying Y (2010) Copper oxide nanoparticles and ionic liquid modified carbon electrode for the non-enzymatic electrochemical sensing of hydrogen peroxide. Microchim Acta 171:117–123
Weekes J, Lam AKY, Sebesan S, Ho YH (2009) Irinotecan therapy and molecular targets in colorectal cancer: a systemic review. World J Gastroenterol 15:3597–3602
Glimelius B (2005) Benefit-risk assessment of irinotecan in advanced colorectal cancer. Drug Saf 28:417–433
Thomas CJ, Rahier NJ, Hecht SM (2004) Camptothecin: current perspectives. Bioorg Med Chem 12:1585–1604
Bobenicová M, Valko M, Brezová V, Dvoranová D (2014) UVA generated free radicals in irinotecan (CPT-11) in the presence of copper ions. J Photochem Photobiol A Chem 290:125–138
(2015) British national formulary: BNF 69 (69 ed.). British Medical Association, p 624. ISBN 9780857111562
Mohammadi A, Esmaeili F, Dinarvand R, Atyabi F, Walker RB (2010) Simultaneous determination of irinotecan hydrochloride and its related compounds by high performance liquid chromatography using ultraviolet detection. Asian J Chem 22:3966–3972
Baylatry MT, Joly AC, Pelage JP, Lefevre LB, Prugnaud JL, Laurent A, Fernandez C (2010) Simple liquid chromatography method for the quantification of irinotecan and SN38 in sheep plasma: application to in vivo pharmacokinetics after pulmonary artery chemoembolization using drug eluting beads. J Chromatogr B 878:738–742
Caceres MIR, Meras I, Soto NEO, de Alb PLL, Martınez LL (2008) Spectrofluorimetric determination of irinotecan in the presence of oxidant agents and metal ions. Talanta 74:1484–1491
Sharma S, Sharma MC (2016) Development and validation of spectrophotometric method and TLC densitometric determination of irinotecan HCl in pharmaceutical dosage forms. Arab J Chem 9:S1368–S1372
Serrano LA, Yang Y, Salvati E, Stellacci F, Krol S, Guldin S (2018) pH-mediated molecular differentiation for fluorimetric quantification of chemotherapeutic drugs in human plasma. Chem Commun 54:1485–1488
Temerk Y, Ibrahim M, Ibrahim H, Schuhmann W (2018) Comparative studies on the interaction of anticancer drug irinotecan with dsDNA and ssDNA. RSC Adv 8:25387–25395
Norouzi P, Qomi M, Nemati A, Ganjali MR (2009) Determination of anticolon cancer drug, irinotecan by fast fourier transforms continuous cyclic voltammetry. Int J Electrochem Sci 4:1248–1261
Temerk YM, Ibrahim HSM (2013) Individual and simultaneous square wave voltammetric determination of the anticancer drugs emodin and irinotecan at renewable pencil graphite electrodes. J Braz Chem Soc 24:1669–1678
Karadas N, Sanli S, Akmese B, Topal BD, Can A, Ozkan SA (2013) Analytical application of polymethylene blue-multiwalled carbon nanotubes modified glassy carbon electrode on anticancer drug irinotecan and determination of its ionization constant value. Talanta 115:911–919
Temerk YM, Ibrahim HSM, Schuhmann W (2016) Square wave cathodic adsorptive stripping voltammetric determination of the anticancer drugs flutamide and irinotecan in biological fluids using renewable pencil graphite electrodes. Electroanalysis 28:372–379
Zotti G, Berlin A, Vercelli B (2017) Electrochemistry of conjugated planar anticancer molecules: irinotecan and sunitinib. Electrochim Acta 231:336–343
Bonazza G, Tartaggia S, Toffoli G, Polo F, Daniele S (2018) Voltammetric behaviour of the anticancer drug irinotecan and its metabolites in acetonitrile. Implications for electrochemical therapeutic drug monitoring. Electrochim Acta 289:483–493
Hatamluyi B, Eshaghi Z, Zahed FM, Darroudi M (2019) A novel electrochemical sensor based on GQDs-PANI/ZnO-NCs modified glassy carbon electrode for simultaneous determination of irinotecan and 5-fluorouracil in biological samples. Sensors Actuators B Chem 286:540–549
Jovanovic IN, Lovric SK, Vrdoljak AL, Popovic AR, Neuberg M (2018) Voltammetric characterisation of anticancer drug irinotecan. Electroanalysis 30:336–344
Ibrahim H, Temerk Y (2016) Sensitive electrochemical sensor for simultaneous determination of uric acid and xanthine in human biological fluids based on the nano-boron doped ceria modified glassy carbon paste electrode. J Electroanal Chem 780:176–186
Ibrahim H, Temerk Y, Farhan N (2018) A novel sensor based on nanobiocomposite Au-In2O3-chitosan modified acetylene black paste electrode for sensitive detection of antimycotic ciclopirox olamine. Talanta 179:75–85
Ibrahim H, Temerk Y (2016) A novel electrochemical sensor based on B doped CeO2 nanocubes modified glassy carbon microspheres paste electrode for individual and simultaneous determination of xanthine and hypoxanthine. Sensors Actuators B Chem 232:125–137
Klett J, Hardy R, Romine E, Wells C, Burchell T (2000) High-thermal conductivity, mesophase-pitch-derived carbon foams: effect of precursor on structure and properties. Carbon 38:953–973
Punckt C, Pope MA, Liu YM, Aksay IA (2016) Structure-dependent electrochemistry of reduced graphene oxide monolayers. J Electrochem Soc 163:H491–H498
Goyal RN, Gupta VK, Chatterjee S (2010) Voltammetric biosensors for the determination of paracetamol at carbon nanotube modified pyrolytic graphite electrode. Sensors Actuators B Chem 149:252–258
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101:19–28
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 6300 kb).
Rights and permissions
About this article
Cite this article
Ibrahim, H., Temerk, Y. Gold nanoparticles anchored graphitized carbon nanofibers ionic liquid electrode for ultrasensitive and selective electrochemical sensing of anticancer drug irinotecan. Microchim Acta 187, 579 (2020). https://doi.org/10.1007/s00604-020-04560-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04560-9