Abstract
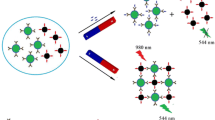
For the first time a competitive immunoassay was developed by employing T-2 antibody-functionalized magnetite nanoparticles and T-2 toxin-conjugated fluorescent quantum dots (QDs). Free T-2 and the T-2-modified QDs compete for binding to antibody-modified magnetic beads; the magnetic beads collected by magnetic separation were subjected to fluorescence intensity analysis (with excitation/emission wavelengths at 460/616 nm). This competitive immunoassay for T-2 toxin determination was applied both in a microcentrifuge tube and on a 96-well plate. The dynamic range of the immunoassay is 1–100 ng mL−1, the limit of detection (LOD) is 0.1 ng mL−1, and determination was completed in about 40 min and 30 min in the microcentrifuge tube and 96-well plate, respectively. Moreover, the biolayer interferometry (BLI) technique was employed for T-2 determination for the first time, in which the conjugate of T-2 toxin and bovine serum albumin (BSA) was immobilized on the sensors before detection. Its average recovery of T-2 toxin from barley sample ranged from 82.00 to 123.33%, and the relative standard deviation (RSD) was between 9.42 and 15.73%. The LOD of the BLI-based assay is 5 ng mL−1, and it only takes 10 min to finish the determination.

Graphical abstract





Similar content being viewed by others
References
Li Y, Luo X, Yang S, Cao X, Wang Z, Shi W, Zhang S (2014) High specific monoclonal antibody production and development of an ELISA method for monitoring T-2 toxin in rice. J Agric Food Chem 62:1492–1497. https://doi.org/10.1021/jf404818r
Lippolis V, Pascale M, Maragos CM, Visconti A (2008) Improvement of determination sensitivity of T-2 and HT-2 toxins using different fluorescent labeling reagents by high-performance liquid chromatography. Talanta 74:1476–1483. https://doi.org/10.1016/j.talanta.2007.09.024
Li Y, Zhang J, Mao X, Wu Y, Liu G, Song L, Li Y, Yang J, You Y, Cao X (2017) High-sensitivity chemiluminescent immunoassay investigation and application for the determination of T-2 toxin and major metabolite HT-2 toxin. J Sci Food Agric 97:818–822. https://doi.org/10.1002/jsfa.7801
Ler SG, Lee FK, Gopalakrishnakone P (2006) Trends in determination of warfare agents-determination methods for ricin. Staphylococcal enterotoxin B and T-2 toxin. J Chromatogr A 1133:1–12. https://doi.org/10.1016/j.chroma.2006.08.078
Zhang M, Wang Y, Yuan S, Sun X, Huo B, Bai J, Peng Y, Ning B, Liu B, Gao Z (2019) Competitive fluorometric assay for the food toxin T-2 by using DNA-modified silver nanoclusters. aptamer-modified magnetic beads. and exponential isothermal amplification. Microchim Acta 186:4. https://doi.org/10.1007/s00604-019-3322-z
Khan IM, Zhao S, Niazi S, Mohsin A, Shoaib M, Duan N, Wu S, Wang Z (2018) Silver nanoclusters based FRET aptasensor for sensitive and selective fluorescent determination of T-2 toxin. Sensors Actuators B Chem 277:328–335. https://doi.org/10.1016/j.snb.2018.09.021
Wang Y, Ning B, Peng Y, Bai J, Liu M, Fan X, Sun Z, Lv Z, Zhou C, Gao Z (2013) Application of suspension array for simultaneous determination of four different mycotoxins in corn and peanut. Biosens Bioelectron 41:391–396. https://doi.org/10.1016/j.bios.2012.08.057
Wang Y, Liu N, Ning B, Liu M, Lv Z, Sun Z, Peng Y, Chen C, Li J, Gao Z (2012) Simultaneous and rapid determination of six different mycotoxins using an immunochip. Biosens Bioelectron 34:44–50 https://doi.org/10.1016/j.bios.2011.12.057
Rai M, Jogee PS, Ingle AP (2015) Emerging nanotechnology for determination of mycotoxins in food and feed. Int J Food Sci Nutr 66:363–370. https://doi.org/10.3109/09637486.2015.1034251
Zhang M, Huo B, Yuan S, Ning B, Bai J, Peng Y, Liu B, Gao Z (2018) Ultrasensitive determination of T-2 toxin in food based on bio-barcode and rolling circle amplification. Anal Chim Acta 1043:98–106. https://doi.org/10.1016/j.aca.2018.09.007
Dong M, Si W, Wang W, Bai B, Nie D, Song W, Zhao Z, Guo Y, Han Z (2016) Determination of type A trichothecenes in coix seed by magnetic solid-phase extraction based on magnetic multi-walled carbon nanotubes coupled with ultra-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 408:6823–6831. https://doi.org/10.1007/s00216-016-9809-0
Foubert A, Beloglazova NV, Saeger SD (2017) Comparative study of colloidal gold and quantum dots as labels for multiplex screening tests for multi-mycotoxin determination. Anal Chim Acta 955:48–57. https://doi.org/10.1016/j.aca.2016.11.042
Malhotra BD, Srivastava S, Ali MA, Singh C (2014) Nanomaterial-based biosensors for food toxin determination. Appl Biochem Biotechnol 174:880–896. https://doi.org/10.1007/s12010-014-0993-0
Reverté L, Prieto-Simón B, Campàs M (2016) New advances in electrochemical biosensors for the determination of toxins: nanomaterials. Magnetic beads and microfluidics systems. A review. Anal Chim Acta 908:8–21. https://doi.org/10.1016/j.aca.2015.11.050
Qie Z, Yan W, Gao Z, Meng W, Xiao R, Wang S (2019) Ovalbumin antibody-based fluorometric immunochromatographic lateral flow assay using CdSe/ZnS quantum dot beads as label for determination of T-2 toxin. Microchim Acta 186:12. https://doi.org/10.1007/s00604-019-3964-x
Ding G, Wang Y, Guo Y, Xu L (2014) Integrin αVβ3-targeted magnetic nanohybrids with enhanced antitumor efficacy, cell cycle arrest ability, and encouraging anti- cell- migration activity. ACS Appl Mater Interfaces 6:16643–16652. https://doi.org/10.1021/am503359g
Gao X, Cui Y, Levenson RM, Chung LWK, Nie S (2004) In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol 22:969–976. https://doi.org/10.1038/nbt994
Concepcion J, Witte K, Wartchow C, Choo S, Yao D, Persson H, Wei J, Li P, Heidecker B, Ma W, Varma R, Zhao LS, Perillat D, Carricato G, Recknor M, Du K, Ho H, Ellis T, Gamez J, Howes M, Phi-Wilson J, Lockard S, Zuk R, Tan H (2009) Label-free determination of biomolecular interactions using biolayer interferometry for kinetic characterization. Comb Chem High Throughput Screen 12:791–800. https://doi.org/10.2174/138620709789104915
Mechaly A, Cohen H, Cohen O, Mazor O (2016) A biolayer interferometry-based assay for rapid and highly sensitive determination of biowarfare agents. Anal Biochem 506:22–27. https://doi.org/10.1016/j.ab.2016.04.018
Gao S, Zheng X, Wu J (2018) A biolayer interferometry-based enzyme-linked aptamer sorbent assay for real-time and highly sensitive determination of PDGF-BB. Biosens Bioelectron 102:57–62. https://doi.org/10.1016/j.bios.2017.11.017
Gao S, Zheng X, Hu B, Sun M, Wu J, Jiao B, Wang L (2017) Enzyme-linked. Aptamer-based. Competitive biolayer interferometry biosensor for palytoxin. Biosens Bioelectron 89:952–958. https://doi.org/10.1016/j.bios.2016.09.085
Guo Y, Li C, Shen H, Zhang X, Li L, Yu Q, Xu L (2011) RGDC peptide modified quantum dots labelling and imaging of tumor cells. Chem Res Chin Univ 27:832–835. http://crcu.jlu.edu.cn/EN/Y2011/V27/I5/832
Abozaid RM, Lazarević ZŽ, Radović I, Gilić M, Šević D, Rabasović MS, Radojević V (2019) Optical properties and fluorescence of quantum dots CdSe/ZnS-PMMA composite films with interface modifications. Opt Mater 92:405–410. https://doi.org/10.1016/j.optmat.2019.05.012
Laurent S, Forge D, Port M, Roch A, Robic C, Elst LV, Muller RN (2008) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 108:2064–2110. https://doi.org/10.1021/cr068445e
Du P, Jin M, Zhang C, Chen G, Cui X, Zhang Y, Zhang Y, Zou P, Jiang Z, Cao X, She Y, Jin F, Wang J (2018) Highly sensitive determination of triazophos pesticide using a novel bio-bar-code amplification competitive immunoassay in a micro well plate-based platform. Sensors Actuators B Chem 256:457–464. https://doi.org/10.1016/j.snb.2017.10.075
Shi H, Schwab W, Yu P (2019) Natural occurrence and co-contamination of twelve mycotoxins in industry-submitted cool-season cereal grains grown under a low heat unit climate condition. Toxins 11:160. https://doi.org/10.3390/toxins11030160
Janssen EM, Liu C, Fels-Klerx HJVD (2018) Fusarium infection and trichothecenes in barley and its comparison with wheat. World Mycotoxin J 11:33–46. https://doi.org/10.3920/WMJ2017.2255
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81771965, 31571017, 81571791, 81271697), the National “Significant New Drug Creation” Science and Technology Major Program (No. 2012ZX09503001-003), the project of Science and Technology Department of Jilin Province, China (No. 20170204027NY, 20130206069GX), and the Special Project of Biological Medicine of Jilin Province, China (No. SXGJSF2017-1).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they have no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary data to this article can be found online at https://doi.org/xx.xxx
ESM 1
(DOC 4626 kb).
Rights and permissions
About this article
Cite this article
Li, Y., Xu, L., Fu, X. et al. A competitive immunoassay based on engineered magnetic/fluorescent nanoparticles and biolayer interferometry-based assay for T-2 toxin determination. Microchim Acta 187, 514 (2020). https://doi.org/10.1007/s00604-020-04493-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04493-3