Abstract
Molybdenum disulfide (MoS2) was supported on graphene oxide (GO) by hydrothermal method. The resulting nanocomposite (MoS2-rGO) was characterized by X-ray photoelectron spectroscopy, scanning electron microscopy, and transmission electron microscopy. The experiments show that at pH 2, MoS2-rGO has a great affinity for adsorption of hexavalent chromium ions while Cr(III) ions remain in aqueous sample. In the adsorption process, the dominant role plays chemisorption. The determined adsorption capacity is 583.5 mg g−1. Parameters affecting the extraction process, namely sample pH, sample volume, contact time, and matrix ions, were investigated by sequential batch tests. Under optimal conditions (pH 2, sample volume 50 mL, sonication time 10 min, adsorbent mass 1 mg), the calibration curve covers the 1–200 ng mL−1 range with a correlation coefficient (R2) of 0.998. The recovery of the method is 97 ± 3%. Other data of merit include a relative standard deviation of < 3.5%, enrichment factor of 3350, and detection limit of 0.050 ng mL−1. The accuracy of the method was confirmed by analysis of the reference materials QC1453 (chromium VI in drinking water) and QC3015 (chromium VI in seawater). The method was successfully applied to chromium speciation in water samples, including high salinity ones. The concentration of Cr(III) was calculated as the difference between the total concentration of chromium (after oxidation of Cr(III) to Cr(VI) with potassium permanganate) and the initial Cr(VI) content.
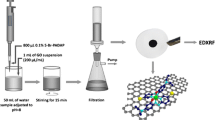
Graphical abstract

Schematic presentation of a method for determination of chromium species by energy dispersive X-ray fluorescence spectrometry after preconcentration on molybdenum disulfide supported on reduced graphene oxide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Determination of chromium species in waters is of prime interest due to their contrasting bioavailability and physiological properties. According to the World Health Organization (WHO) [1] and the United States Environmental Protection Agency (EPA) [2], the permissible level of total chromium content in drinking water is 50 ng mL−1 and 100 ng mL−1, respectively. Most analytical techniques including inductively coupled plasma atomic emission spectrometry (ICP-OES), inductively coupled plasma mass spectrometry (ICP-MS), flame and electrothermal atomic absorption spectrometry (FAAS and ET-AAS), energy dispersive X-ray fluorescence spectrometry (EDXRF), or total reflection X-ray fluorescence spectrometry (TXRF) enable determining total concentration of chromium in environmental samples. Thus, isolation and/or preconcentration step is necessary to determine chromium species.
According to the literature, there are three main procedures for chromium speciation: (i) selective preconcentration of Cr(III) or Cr(VI) and further oxidation or reduction determining its total concentration, (ii) preconcentration of both chromium states at different pH values and subsequent elution with different solvents, or (iii) preconcentration of both forms of chromium at the same sample pH and subsequent elution with different solvents. The first approach is the most popular in chromium speciation.
Among a vast array of sample pretreatment methods used for chromium speciation, solid phase extraction (SPE) and dispersive solid phase extraction (DSPE) have been the most often utilized. Their widespread use results from simplicity, relatively low costs, the ability to process large volume samples, fast and easy phase separation, minimized solvent consumption, possibility of obtaining high enrichment factors, easy coupling with different analytical techniques, and many existing adsorbents [3]. However, classical adsorbents, such as activated carbon [4], modified silica gel [5], chelating resins [6, 7], and Amberlite XAD-4 resin series [8, 9], have been gradually replaced by nanosized adsorbents due to their unique large surface area, high adsorption capacity, and chemical stability. For preconcentration of chromium species from environmental samples, various types of nanomaterials including oxidized multiwalled nanotubes (MWCNTs) [10], MWCNTs and graphene oxide (GO) modified with Aliquat 336 [11, 12], MWCNTs modified with 3-(2-aminoethylamino) propyltrimethoxysilane (AAPTS-MWCNTs) [13], GO decorated with triethylenetetramine-modified magnetite (mf-GO) [14], GO functionalized with 3-(2-aminoethylamino) propyltrimethoxysilane (GO-1N) [15], and molybdenum disulfide (MoS2) [16] were used. MoS2 is the best-known and widely explored representative of the transition metal dichalkogen (TMDs) group. Its crystals consist of hexagonal molybdenum layers disposed between two sulfur layers by covalent bonds. These bonds are responsible for excellent mechanical strength and thermal stability up to 1090 °C in an inert atmosphere environment. Weak van der Waals bonds allow forming thin MoS2 layers of a single or a multi-layer thickness. The presence of a large amount of sulfur atoms on surfaces and edges which act as a soft Lewis base makes MoS2 excellent adsorbent for the removal of heavy metal ions that behave like soft Lewis acids. Its defect-rich structure formed during synthesis together with enlarge interlayer spacing between the three atom layers (S-Mo-S) allows penetrating small molecules or ions into MoS2 layers. It results in increase of both its adsorption capacity and possibility of surface modification [17]. For chromium preconcentration, different composites of MoS2 including magnetic nanoparticles (Fe3O4NPs) decorated with MoS2 (MoS2@Fe3O4) [18], sodium dodecyl sulfate (SDS)-intercalated MoS2 (SDS-MoS2) [19], MoS2 coated Mg/Al layered double hydroxide composite (LDHs@MoS2) [20], polyvinylpyrrolidone (PVP), and polyacrylamide (PAM) intercalated MoS2 composites [21] were successfully applied.
Membrane-based systems for chromium preconcentration were also proposed [22, 23]. Their combination with XRF techniques simplifies procedures by elimination of elution of target ions and centrifugation or filtration step. As a result, the risk of the analyte loss or sample contamination is minimized. The main drawback of such approach is the time required to establish the equilibrium state that is much longer than in the case of DSPE.
Herein, a method for chromium speciation in waters by energy dispersive X-ray fluorescence spectrometry (EDXRF) after preconcentration on molybdenum disulfide supported on reduced graphene oxide (MoS2-rGO) is described. The method is based on a selective adsorption of hexavalent chromium on the MoS2-rGO surface. The concentration of Cr(III) is calculated as the difference between the total concentration of chromium (after oxidation of Cr(III) to Cr(VI) with potassium permanganate) and the initial Cr(VI) content. Considering that the adsorption of Cr(VI) occurs at acidic conditions, the method is resistant to high concentration of other coexisting anions, alkali, and alkaline earth cations usually present in water samples and for that reason can be used for sensitive hexavalent chromium determination and speciation in high salinity samples. The synthesis of MoS2-rGO, its structural characterization, the adsorption process optimization, and validation of the method were carefully studied. The research broadens the scope of MoS2-rGO application since its first successful use for Pb(II) and Ni(II) preconcentration from urine, saliva, and water samples prior to AAS determination [24].
Experimental
Reagents and solutions
Stock solutions of Cr(III), Cr(VI), Se(IV), Se(VI), As(III), and As(V) (1 mg mL−1), tannic acid, nitric acid (65%, Suprapur®), and ammonium hydroxide solution (25%, Suprapur®) were purchased from Merck (Darmstadt, Germany, www.merckgroup.com). Salts used for the interferences studies, potassium permanganate, sulfuric acid, sodium molybdate dihydrate, hydrochloride acid, and ethanol were purchased from POCh (Gliwice, Poland, www.poch.com.pl). Graphite powder (325 mesh) was purchased from Alfa Aesar (Karlsruhe, Germany, www.alfa.com). L-cysteine, humic acid, and certified materials: chromium VI in drinking water (QC1453) and chromium VI in seawater (QC3015), were purchased from Sigma-Aldrich (Laramie, Wyoming, USA, www.sigmaaldrich.com). High-purity water obtained from a Milli-Q system (Millipore, Molsheim, France, www.merckmillipore.com) was applied in the whole studies.
Instruments
X-ray photoelectron spectroscopy measurements were performed with a PHI 5600 Physical Electronic Spectrometer (www.phi.com) with the use of monochromated Al Kα radiation, at the ultra-high vacuum pressure of 5 × 10−10 mbar, 15 kV, and 20 mA. The energy resolution was 0.1 eV. All photoelectron spectra were calibrated against the peaks of Au 4f7/2 at 83.98 eV, Ag 3d5/2at 368.27 eV, and Cu 2p3/2 at 932.67 eV of binding energy. The analysis of the surfaces of the powdered material was carried out at take-off angle 45°. The JEOL-5410 scanning electron microscope (SEM) and JEOL JEM 3010 transmission electron microscope (TEM) (www.jeol.co.jp/en) were used to the MoS2-rGO surface observations. Determination of Cr(VI) and chromium speciation was conducted on an Epsilon 3 EDXRF spectrometer (PANalytical, Almelo, The Netherlands, www.panalytical.com). A spectrometer equipped with a 200-μm Al primary beam filter worked at 30 kV and 0.300 mA, in atmospheric conditions. A counting time of 300 s was used in all EDXRF measurements. Adsorption capacity of MoS2-rGO and recovery research were performed using a SpecroFMS16a spectrometer with excitation in the ICP plasma (Spectro Analytical Instruments, www.spectro.com). A spectrometer worked at the following conditions: plasma power 1.4 kW, coolant gas Ar, 12 L min−1, auxiliary gas Ar, 1 L min−1, nebulizer gas Ar, 1 L min−1, nebulizer pressure 3.2 bar, nebulizer cross-flow type, sample uptake rate 2 mL min−1, wavelength 284.3 nm.
Synthesis of MoS2-rGO
Graphene oxide applied to MoS2-rGO synthesis was prepared by the modified Hummers’ method [25]. Synthesis of MoS2-rGO nanoparticles was conducted according to the literature data [24]. Succinctly summarizing, the 25 mL of aqueous suspension containing 0.1 g of GO was stirred at 900 rpm for 6 h at room temperature. Subsequently, 0.5 g of Na2MoO4·2H2O was added to the mixture and stirring was continued for the next hour. Then, suspension pH was adjusted to 6.5, and the solution containing 1 g of L-cysteine in 50 mL of water was added. The resulting mixture was transferred into a 100-mL Teflon-lined stainless steel autoclave and heated at 180 °C for 36 h in a laboratory dryer. The synthesized MoS2-rGO in the form of black precipitate was separated by centrifugation, washed 10 times with ethanol and water in order to remove an excess of L-cysteine and Na2MoO4·2H2O, and then dried at 80 °C. The scheme of MoS2-rGO synthesis is shown in Fig. 1.
Batch adsorption studies for Cr(VI)
To 25 mL in volume aqueous samples containing appropriate amount of Cr(VI) ions, 1 mg of MoS2-rGO was added, and after pH adjustment to 2, the resulting suspensions were stirred at 900 rpm for 3 h at room temperature. Next, the aqueous suspensions were filtered with 0.22-μm membrane filters, and filtrates were collected in test tubes for further ICP-OES measurements. The metal ion concentration adsorbed on the MoS2-rGO surface (mg g−1) was calculated using the following equation: qe = [(Co-Ce)·V]/madsorbent, where Co is the initial concentration of Cr(VI) in aqueous solution (mg L−1), Ce is the equilibrium concentration (mg L−1), V is a suspension volume, and madsorbent is MoS2-rGO mass (mg).
Ultrasound-assisted dispersive micro-solid phase extraction for Cr(VI)
One milligram of MoS2-rGO was added to 50 mL of aqueous sample containing different concentrations of Cr(VI) ions. Then, the pH was adjusted to 2 with 0.1 mol L−1 HNO3, and subsequently, the suspension was sonicated for 10 min. In the next step, the sample was filtered through a membrane filter (0.22 μm) under reduced pressure with the use of a customized filtration assembly of 5-mm diameter. The filter covered with MoS2-rGO and adsorbed Cr(VI) ions was dried under air conditions prior to EDXRF measurement. The blank sample was prepared in the same way as described above, but high-purity water was used instead of the examined sample.
Chromium speciation
In order to carry out the speciation analysis, two aliquots of a sample are required. The first aliquot is analyzed for the Cr(VI) content, while the second one for the total chromium concentration determined after the oxidation of Cr(III) ions to Cr(VI) with potassium permanganate [26]. The procedure was as follows: 4–5 drops of 0.02 mol L−1 KMnO4 were added to 50 mL of a sample at pH 2. Then, the solution was heated for 15 min under cover at 90–95 °C. After cooling the sample, preconcentration of Cr(VI) was performed according to the procedure described above. The Cr(III) amount was calculated as the difference between the total chromium and initial Cr(VI) content.
Real samples and sample preparation
Lake, spring, and river waters, collected in the Upper Silesian region (Poland), were filtered through a 0.22-μm cellulose acetate membrane (Millipore), and after acidification with HNO3 stored without access of light at 4 °C. The artificial sea water was prepared by dissolving 21.03 g NaCl, 3.52 g Na2SO4, 0.61 g KCl, 0.088 g KBr, 0.034 g Na2B4O7 ∙ 10H2O, 9.50 g MgCl2 ∙ 6H2O, 1.32 g CaCl2 ∙ 2H2O, 0.02 g SrCl2 ∙ 6 H2O, and 0.02 g NaHCO3 in 1 L of high-purity water [27].
Results and discussion
Characterization of MoS2-rGO
The synthesized composite was characterized by XPS. Figure 2 shows the survey spectrum of MoS2-rGO consisted of carbon, oxygen, sulfur, and molybdenum states which is in accordance with its composition. The additional Na 2s and Cl 2p states are typical for human sweat.
Figure 3 shows the high-resolution C1s, Mo3d, S2p, and O1s spectra of MoS2-rGO. The C1s spectrum was deconvoluted into four peaks at 284.5 (C-C), 285.6 (C-S), 286.2 (C=O), and 288.7 (O-C=O) eV. The Mo3d spectrum of MoS2-rGO shows not only two doublets Mo4+ (3d5/2 at 228.5 eV, 3d3/2 at 232.5 eV) and Mo5+ (3d5/2 at 230.5 eV, 3d3/2 at 235.2 eV) but also S2s state at 226 eV. The S2p of MoS2-rGO reveals four peaks at 162.2 eV(S2− 2p3/2), 163.3 eV (S2− 2p1/2), 164.7 eV (S22−), and 168.5 eV (S4+). The O1s of MoS2-rGO reveals four peaks at 531.5, 533.4, 534.3, and 537 eV assigned to C=O, Mo-O/S-O, C-OH, and O-C=O. Those changes of shape and intensity at the maximum position of C1s peaks involve successful modification of the material. Mo3d, S2p, S2s, and also both C1s and O1s deconvoluted lines indicate the functionalization of GO. It can be seen that new peaks (C–S in carbon line and Mo-O/S-O in oxygen line) assigned to surface groups are present in the spectra. The location of main peaks on the deconvoluted XPS spectra is in a good agreement with those described in the literature data [28].
The modification of graphene oxide surface with molybdenum disulfide nanoparticles was also confirmed by the EDXRF technique. The presence of sulfur (overlapped Kα at 2.31 keV and Kβ at 2.46 keV) and molybdenum (Kα at 17.48 keV and Kβ at 19.61 keV) peaks in the recorded spectrum (Fig. 4) proves successful decoration of MoS2 on GO surface.
The SEM and TEM images of MoS2-rGO surface at different magnifications are presented in Fig. 5. The strongly undulating surface of the nanomaterial indicates a large surface area of a single flake which may result in a high extractive capacity.
Optimization of ultrasound-assisted dispersive micro-solid phase extraction for Cr(VI)
The following parameters were optimized: (a) pH value; (b) sample volume; (c) contact time; (d) adsorbent mass. Respective text and figures on optimizations are given in the Electronic Supporting Material. In short, the following experimental conditions were found to give best results: (a) pH value, 2; (b) sample volume, 50 mL; (c) contact time, 10 min; (d) adsorbent mass, 1 mg.
Adsorption isotherms for Cr(VI)
In order to clarify the adsorption mechanism of Cr(VI) ions on MoS2-rGO surface, the Langmuir [29] and Freundlich [30] isotherm models were employed. The isotherms were computed using the following equations:
where qmax is the maximum amount of Cr(VI) ions adsorbed on 1 mg of MoS2-rGO surface to form a single layer coverage at highest equilibrium ion concentration (mg g−1), KL is the adsorption enthalpy (L mg−1), KF (mg1-n Ln g−1) and n are Freundlich constants related to the adsorption facility and adsorption intensity, respectively.
The course of adsorption isotherms as well as parameters attained by fitting the experimental data to the Langmuir and Freundlich isotherm models is presented in Fig. S4. As can be seen, better experimental data fitting was obtained for the Langmuir isotherm model. It seems that in the tested system, the monolayer coverage is more advantageous than the multiple adsorption process. Thus, the adsorption is of chemisorption nature, which involves the formation of coordination bonds between the Cr(VI) ions and S atoms present on the surface of MoS2-rGO. The calculated n value from the Freundlich isotherm model points out the dominant role of the adsorption process [31]. In Table S1, the adsorption capacities of MoS2-based nanomaterials used for the adsorption of Cr(VI) from the aqueous samples are presented. It can be noted that the MoS2-rGO exhibits the highest affinity to Cr(VI) ions. The qmax value is nearly 6.5 times higher than that reported for the raw MoS2.
Study of potential interferences
The key to practical application of the method is to study the influence of potentially interfering ions and organic matter present in surface water on the Cr(VI) ions recovery. The natural organic matter (NOM) of waters originates from plants and animal decomposition products. Typically, NOM concentration in surface water cover the 0.1–20 mg L−1 range. NOM consists of non-humic substances easily decomposed by microorganism and humic substances (HS) more stable than their forerunner. HS including fulvic acids, humic acids, and humins vary in a molecular weight, structure, composition, and positions of functional groups. Despite the differences, all contain carboxyl, phenol, hydroxyl, amine, and quinine groups [32]. In order to study the influence of NOM on the Cr(VI) adsorption, humic acid (HA) and tannic acid (TA) were used as model compounds.
Sample solutions containing 20 ng mL−1 of Cr(VI) and various amounts of hypothetically interfering species were prepared, and the preconcentration procedure was performed. If the scope of changes in the recovery was within the range R ± 5%, it was assumed that the tested ion does not affect the results. The achieved results are summarized in Table S2.
The conducted studies reveal that the most typical anions (Cl−, SO42−, NO3−, CO32−, HCO3−, PO43−, HPO42− Br−, B4O72−) and cations (Na+, K+, Ca2+, Mg2+, Al3+) do not influence the adsorption of Cr(VI) on the MoS2-rGO surface. The transition metals do not affect the chromium determination at amounts lower or equal 500-fold excess over Cr(VI) ions. In the case of cations, it results mainly from the electrostatic repulsion between two positively charged specimens. In a view of the soft and hard acids and base theory, sulfur as soft base weakly interacts with alkali and alkaline earth cations which are considered to be hard acids. The resistance to high concentrations of the anions can be partly explained by weaker interaction with positively charged adsorbent surface unlike oxoions of Cr(VI). The presence of humic and tannic acids does not affect the chromium recovery at amounts lower or equal to 250-fold excess over Cr(VI) ions. The conducted studies demonstrated that the method can be applied in the analysis of water samples, even high salinity ones.
Analytical characteristics
To characterize the described method for hexavalent chromium determination, some parameters, namely (i) linearity range, (ii) detection and quantification limits, (iii) precision at two concentrations of Cr(VI) ions, (iv) enrichment factor, and (v) recovery, were determined. The proportional relationship between the fluorescent radiation intensity and the concentration of the analyte ions was achieved for the concentration range of 1–200 ng mL−1 (R2 = 0.998).
Based on the following equation: LOD = (3/k)(B/t)1/2, where k is the count sensitivity (s−1 μg−1), B is the count rate of blank sample (counts s−1), and t is the counting time (s), the LOD value was 0.050 ng mL−1. The limit of quantification (LOQ) was computed by multiplying LOD by 3.3 and equaled 0.165 ng mL−1. The LOD and LOQ were nearly 1000-fold lower than those for the direct EDXRF analysis of water samples (with the counting time in the 3–30 min range using 100–1000 mL in volume samples). The LOD and LOQ values are much lower than the acceptable concentration of Cr (50 ng mL−1) for drinking water suggested by the World Health Organization (WHO) [1]. The precision of the method determined for six replicates at two concentrations, namely 5 ng mL−1 and 50 ng mL−1, were 3.5 and 1.8%, respectively. The enrichment factor (EF) was calculated as ratio of sensitivity of the DMSPE–EDXRF procedure to the sensitivity of the direct EDXRF measurement. The EF of 3350 is much higher than the typical EF values of FAAS or ICP-OES procedures. It can be explained by the absence of the elution of the analyte from the adsorbent surface. Recovery value of 97 ± 3 for six replicates and chromium concentration of 20 ng mL−1 was calculated from the following formula: R = ((cinitial-cDMSPE)/cinitial) × 100%, where R is recovery, cinitial is concentration of Cr(VI) added to the solution (μg L−1), cDMSPE is the concentration of Cr(VI) determined after DMSPE procedure (μg L−1).
Comparison of the method for hexavalent chromium determination with the literature data is shown in Table 1. Although FAAS technique is usually considered as favorable due to low costs, availability, operational equipment, and high sample throughput, the LOD for chromium are moderate even after preconcentration step [14, 33]. The best LOD is achieved by combination of microcolumn SPE with ICP-MS. Nevertheless, the high operating costs associated with Ar consumption and operating complexity make it expensive in routine analysis [13]. Due to the fact that both techniques operate on liquid samples analyses, elution step prior to measurements is necessary. This not only extends sample pretreatment time but also may be a source of errors related to analyte loss and sample contamination. Different techniques associated mainly with solid samples have also been used for chromium determination, e.g., TXRF [11], wavelength dispersive X-ray fluorescence spectrometry (WDXRF) [12], and EDXRF [15]. It seems that the preconcentration step based on DSPE with nanosized materials applied as adsorbents significantly broadens their practical application to liquid samples. The very small size of such nanomaterials, as well as the use of small amounts of adsorbents (up to 1 mg) guaranteeing thin samples, simplifies quantitative analysis. In that case, particle size effects and matrix effects can be neglected. XRF analysis of hexavalent chromium was preceded by DSPE using MWCNTs modified with Aliquat 336 [11], thin film of Aliquat 336 supported on GO [12], and GO modified with 3-(2-aminoethylamino) propyltrimethoxysilane (GO-1N) [15]. The LOD range from 0.17 to 3 ng mL−1 and they are from 3.4 times to 60 times worse than those for MoS2-rGO. The lack of gas consumption for EDXRF measurements and the elution step elimination are the main benefits, when compared with approaches based on the combination of DSPE with ICP-MS or FAAS.
Regeneration of MoS2-rGO
From the environmental and economic point of view, MoS2-rGO regenerative study is of a key importance. The efficiency of the desorption process was carried out using 2 mL of sodium hydroxide and ammonium hydroxide at a concentration of 1 mol L−1. Such eluents were chosen because in the alkaline solution, the deprotonation of surface functional groups is causing an increase in the negative charge on the surface of MoS2-rGO and thus increases the desorption of Cr(VI). The desorption percentage obtained for NaOH is 98%, while for NH3aq, it does not exceed 80%. Further experiments were carried out using sodium hydroxide as the eluent. The influence of MoS2-rGO surface regeneration cycles on the adsorption of Cr(VI) ions was repeated 5 times. The results presented in Fig. S5 show that as the number of cycles increases, the desorption percentage slowly decreases. For the first two adsorption-desorption cycles, no significant decrease in adsorption properties was observed. After 5 cycles, adsorption decreases to 85%. It can be partially ascribed to the adsorbent loss during the regeneration process. It should be mentioned here that a single experiment was carried out using only 1 mg of the adsorbent. The studies show the potential of MoS2-rGO as an efficient and recyclable nanomaterial for Cr (VI) adsorption from aqueous samples.
Determination of chromium species in waters
To demonstrate usefulness of the MoS2-rGO to chromium speciation in waters, a sample batch spiked with a known concentration of target analyte ions was prepared. The experiments were conducted according to the “Ultrasound-assisted dispersive micro-solid phase extraction for Cr(VI)” and “Chromium speciation” sections. Cr(III) concentration was computed from the difference between the total amount of Cr(VI) (after oxidation of Cr(III) to Cr(VI) with potassium permanganate) and initial content of Cr(VI) ions. The results listed in Table 2 show the potential of the described method to speciation analysis of chromium at trace and ultratrace levels.
In order to check the accuracy of the method, analyses of two certified materials, e.g., chromium VI in drinking water (QC1453) and chromium VI in sea water (QC3015), were also carried out. The results, presented in Table 3, are consistent with the certified values, which proves the utility of the method.
Conclusions
Reduced graphene oxide decorated with MoS2 was applied for the selective preconcentration of Cr(VI) ions from aqueous samples in the acidic media. The strong affinity of a target analyte to the MoS2-rGO nanoparticles results from both electrostatic interaction and outsphere surface complexation. The adsorption capacity of 583.5 mg g−1 is almost 6.5 times higher than that reported for the raw MoS2. A better description of experimental data to the Langmuir isotherm model suggests that in the studied system, chemisorption occurs. The method can be used not only for a sensitive determination of hexavalent chromium but also for chromium speciation. The conducted studies have demonstrated a great potential of the method to water sample analyses even in the case of high salinity ones. Choosing EDXRF as the measurement technique, as an alternative to commonly applied ICP-OES, ICP-MS, or FAAS, eliminates the necessity of elution step which minimizes the risk of the analyte loss or contamination of a sample. Unlike the more generally used spectroscopic techniques, EDXRF does not require gases for carrying out the measurements, which makes the method less expensive and environmental friendly. Although the detection limits offered by the EDXRF technique are not sufficient for trace or ultratrace analyses, the DMSPE/EDXRF procedure being the scope of the work combines the essential advantages of the abovementioned procedures, which broadens the scope of its possible applications.
References
World Health Organization (2011) Guidelines for drinking-water quality. http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/. Accessed 8 Oct 2019
U.S. EPA (2017) Chromium in drinking water. https://www.epa.gov/sdwa/chromium-drinking-water. Accessed 10 Feb 2020
Camel V (2003) Solid phase extraction of trace elements. Spectrochim Acta B 58:1177–1233. https://doi.org/10.1016/S0584-8547(03)00072-7
Gil RA, Cerutti S, Gasquez JA, Olsina RA, Martinez LD (2006) Preconcentration and speciation of chromium in drinking water samples by coupling of on-line sorption on activated carbon to ETAAS determination. Talanta 68:1065–1070. https://doi.org/10.1016/j.talanta.2005.06.069
Bartyzel A, Cukrowska EM (2011) Solid phase extraction method for the separation and determination of chromium(III) in the presence of chromium(VI) using silica gel modified by N,N′-bis-(α-methylsalicylidene)-2,2-dimethyl-1,3-propanediimine. Anal Chim Acta 707:204–209. https://doi.org/10.1016/j.aca.2011.09.023
Tiwari S, Sharma N, Saxena R (2016) On-line speciation of chromium using a modified chelating resin and determination in industrial water samples by flame atomic absorption spectrometry. New J Chem 40:1412–1419. https://doi.org/10.1039/C5NJ02283E
Sacmaci S, Kartal S, Yılmaz Y, Sacmaci M, Soykan C (2012) A new chelating resin: synthesis, characterization and application for speciation of chromium (III)/(VI) species. Chem Eng J 181-182:746–753. https://doi.org/10.1016/j.cej.2011.11.057
Aksoy E, Elçi ŞG, Siyal AN, Elçi L (2018) Chromium speciation using an aminated Amberlite XAD-4 resin column combined with microsample injection-flame atomic absorption spectrometry. Acta Chim Slov 65:512–520. https://doi.org/10.17344/acsi.2017.3984
Narin I, Kars A, Soylak M (2008) A novel solid phase extraction procedure on Amberlite XAD-1180 for speciation of Cr(III), Cr(VI) and total chromium in environmental and pharmaceutical samples. J Hazard Mater 150:453–458. https://doi.org/10.1016/j.jhazmat.2007.04.125
Yu H, Sun W, Zhu X, Zhu X, Wei J (2012) Study on multi-walled carbon nanotubes on-line separation/preconcentration of chromium(III) and chromium speciation. Anal Sci 28:1219–1224. https://doi.org/10.2116/analsci.28.1219
Bahadir Z, Bulut VN, Hidalgo M, Soylak M, Marguí E (2015) Determination of trace amounts of hexavalent chromium in drinking waters by dispersive microsolid-phase extraction using modified multiwalled carbon nanotubes combined with total reflection X-ray fluorescence spectrometry. Spectrochim Acta B 107:170–177. https://doi.org/10.1016/j.sab.2015.03.010
Fernández L, Baldo MF, Messina G, Bertolino FA, Raba J, Aranda PR (2018) Graphene-based materials as solid phase extraction sorbent for chromium (VI) determination in red wine. Microchem J 141:418–422. https://doi.org/10.1016/j.microc.2018.05.043
Peng H, Zhang N, He M, Chen B, Hu B (2015) Simultaneous speciation analysis of inorganic arsenic, chromium and selenium in environmental waters by 3-(2-aminoethylamino)propyltrimethoxysilane modified multi-wall carbon nanotubes packed microcolumn solid phase extraction and ICP-MS. Talanta 131:266–272. https://doi.org/10.1016/j.talanta.2014.07.054
Islam A, Ahmad H, Zaidi N, Kumar S (2016) A graphene oxide decorated with triethylenetetramine-modified magnetite for separation of chromium species prior to their sequential speciation and determination via FAAS. Microchim Acta 183:289–296. https://doi.org/10.1007/s00604-015-1641-2
Janik P, Zawisza B, Talik E, Sitko R (2018) Selective adsorption and determination of hexavalent chromium ions using graphene oxide modified with amino silanes. Microchim Acta 185:117–124. https://doi.org/10.1007/s00604-017-2640-2
Sun H, Wu T, Zhang Y, Ng DHL, Wang G (2018) Structure-enhanced removal of Cr(VI) in aqueous solutions using MoS2 ultrathin nanosheets. New J Chem 42:9006–9015. https://doi.org/10.1039/C8NJ01062E
Wang Z, Mi B (2017) Environmental applications of 2D molybdenum disulfide (MoS2) nanosheets. Environ Sci Technol 51:8229–8244. https://doi.org/10.1021/acs.est.7b01466
Kumar ASK, Jiang S-J, Warchoł JK (2017) Synthesis and characterization of two-dimensional transition metal dichalcogenide magnetic MoS2@Fe3O4 nanoparticles for adsorption of Cr(VI)/Cr(III). ACS Omega 2:6187–6200. https://doi.org/10.1021/acsomega.7b00757
Wang J, Zhang R, Huo Y, Ai Y, Gu P, Wang X, Li Q, Yu S, Chen Y, Yu Z, Chen J, Wang X (2019) Efficient elimination of Cr(VI) from aqueous solutions using sodium dodecyl sulfate intercalated molybdenum disulfide. Ecotoxicol Environ Saf 175:251–262. https://doi.org/10.1016/j.ecoenv.2019.03.064
Wang J, Wang P, Wang H, Dong J, Chen W, Wang X, Wang S, Hayat T, Alsaedi A, Wang X (2017) Preparation of molybdenum disulfide coated Mg/Al layered double hydroxide composites for efficient removal of chromium (VI). ACS Sustain Chem Eng 5:7165–7174. https://doi.org/10.1021/acssuschemeng.7b01347
Wang J, Wang X, Zhao G, Song G, Chen D, Chen H, Xie J, Hayat T, Alsaedi A, Wang X (2018) Polyvinylpyrrolidone and polyacrylamide intercalated molybdenum disulfide as adsorbents for enhanced removal of chromium(VI) from aqueous solutions. Chem Eng J 334:569–578. https://doi.org/10.1016/j.cej.2017.10.068
Romero V, Costas-Mora I, Lavilla I, Bendicho C (2016) Graphene membranes as novel preconcentration platforms for chromium speciation by total reflection X-ray fluorescence. RSC Adv 6:669–676. https://doi.org/10.1039/C5RA23116G
Jamroz E, Kocot K, Zawisza B, Talik E, Gagor A, Sitko R (2019) A green analytical method for ultratrace determination of hexavalent chromium ions based on micro-solid phase extraction using amino-silanized cellulose membranes. Microchem J 149:104060–104068. https://doi.org/10.1016/j.microc.2019.104060
Aghagoli MJ, Shemirani F (2017) Hybrid nanosheets composed of molybdenum disulfide and reduced graphene oxide for enhanced solid phase extraction of Pb(II) and Ni(II). Microchim Acta 184:237–244. https://doi.org/10.1007/s00604-016-2000-7
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814. https://doi.org/10.1021/nn1006368
Tahmasebi Z, Davarani SSH (2016) Selective and sensitive speciation analysis of Cr(VI) and Cr(III), at sub-μg L−1 levels in water samples by electrothermal atomic absorption spectrometry after electro membrane extraction. Talanta 161:640–646. https://doi.org/10.1016/j.talanta.2016.09.016
Kester DR, Duedall IW, Connors DN, Pytkowicz RM (1967) Preparation of artificial seawater. Limnol Oceanogr 12:176–179. https://doi.org/10.4319/lo.1967.12.1.0176
Choi M, Koppala SK, Yoon D, Hwang J, Kim SM, Kim J (2016) A route to synthesis molybdenum disulfide-reduced graphene oxide (MoS2-RGO) composites using supercritical methanol and their enhanced electrochemical performance for Li-ion batteries. J Power Sources 309:202–211. https://doi.org/10.1016/j.jpowsour.2016.01.081
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295. https://doi.org/10.1021/ja02268a002
Freundlich H, Heller W (1939) The adsorption of cis- and trans-azobenzene. J Am Chem Soc 61:2228–2230. https://doi.org/10.1021/ja01877a071
Hameed BH (2008) Equilibrium and kinetic studies of methyl violet sorption by agricultural waste. J Hazard Mater 154:204–212. https://doi.org/10.1016/j.jhazmat.2007.10.010
Tang WW, Zeng GM, Gong JL, Liang J, Xu P, Zhang C, Huang BB (2014) Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: a review. Sci Total Environ 468-469:1014–1027. https://doi.org/10.1016/j.scitotenv.2013.09.044
Qia X, Gao S, Ding G, Tang AN (2017) Synthesis of surface Cr(VI)-imprinted magnetic nanoparticles for selective dispersive solid-phase extraction and determination of Cr (VI) in water samples. Talanta 162:345–353. https://doi.org/10.1016/j.talanta.2016.10.040
Sereshti H, Farahani MV, Baghdadi M (2016) Trace determination of chromium(VI) in environmental water samples using innovative thermally reduced graphene (TRG) modified SiO2 adsorbent for solid phase extraction and UV-vis spectrophotometry. Talanta 146:662–669. https://doi.org/10.1016/j.talanta.2015.06.051
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Conflict of interest
The authors declare that they have no competing of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1799 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pytlakowska, K., Kocot, K., Pilch, M. et al. Ultrasound-assisted dispersive micro-solid phase extraction using molybdenum disulfide supported on reduced graphene oxide for energy dispersive X-ray fluorescence spectrometric determination of chromium species in water. Microchim Acta 187, 542 (2020). https://doi.org/10.1007/s00604-020-04476-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04476-4