Abstract
A detailed study has been carried out on monohydroxycucurbit[7]uril-based stir-bar sorptive extraction (SBSE). A polydimethylsiloxane coating was produced by a sol–gel technique and doped with monohydroxycucurbit[7]uril ((HO)1Q [7]) as a selective sorbent phase. (HO)1Q [7] was chemically bound to the sol–gel silica substrate through hydrolysis and polycondensation. The coating possesses a porous surface, shows strong solvent resistance and good thermal stability, and has a long lifespan. Four groups of compounds, with polarities ranging from apolar polycyclic aromatic hydrocarbons to polar ketones, aromatic amines and phenols, were selected as test analytes. They were extracted with the coated stir bar, then desorbed with methanol and quantified by high-performance liquid chromatography with ultraviolet detection. The limits of detection range between 1.3 and 15 μg L−1, the linear ranges extend from 5 to 10,000 μg L−1, and the relative recoveries from spiked samples range between 76.4 and 97.9%. The intraday relative standard deviations range from 2.3 to 8.6% (for n = 3, at 500 μg L−1). Compared with a commercial PDMS-coated stir bar and a polyether sulfone-coated stir bar, the new stir bar shows wider applicability and better extraction efficiency for each group of compounds. In addition, the stir bar can simultaneously extract mixtures of chemicals of different polarities. This endows it with the potential for recovering a broad group of polar organic compounds.

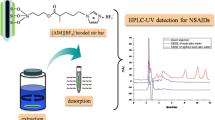
A stir bar for sorptive extraction based on monohydroxycucurbit[7]uril has been prepared by a sol–gel technique. The stir bar coupled with HPLC with UV detection allows for the determination of a variety of polar compounds and multicomponent mixtures, respectively.




Similar content being viewed by others
References
He M, Chen B, Hu B (2013) Recent development in stir bar sorptive extraction. Anal Bioanal Chem 406:2001–2026
Gilart N, Marce RM, Borrull F, Fontanals N (2014) Coatings for stir bar sorptive extraction of polar emerging organic contaminants. TrAC Trends Anal Chem 54:11–23
Faraji H, Husain SW, Helalizadeh M (2011) β-cyclodextrin-bonded silica particles as novel sorbent for stir bar sorptive extraction of phenolic compounds. J Chromatogr Sci 49:482–487
Huang X, Lin J, Yuan D (2011) Preparation of cation-extrange stir bar sorptive extraction based on monolithic material and its application to the analysis of soluble cations in milk by ion chromatography. Analyst 36:4289–4294
Hu C, He M, Chen B, Zhong C, Hu B (2013) Polydimethylsiloxane/metal-organic frameworks coated stir bar sorptive extraction coupled to high performance liquid chromatography-ultraviolet detector for the determination of estrogens in environmental water samples. J Chromatogr A 1310:21–30
Neng NR, Pinto ML, Pires J, Nogueira JMF (2007) Development, optimization and application of polyurethane foams as new polymeric phase for stir bar sorptive extraction. J Chromatogr A 1171:8–14
David F, Ochiai N, Sandra P (2019) Two decades of stir bar sorptive extraction: a retrospective and future outlook. TrAC Trends Anal Chem 112:102–111
Zeng J, Zhao C, Chen J, Subhan F, Luo L, Yu J, Cui B, Xing W, Chen X, Yan Z (2014) Ordered mesoporous carbaon/Nafion as a versatile and selective solid-phase microextraction coating. J Chromatogr A 1365:29–34
Chong SL, Wang DX, Hayes JD, Wilhite BW, Malik A (1997) Sol-gel coating technology for the preparation of solid-phase microextraction fibers of enhanced thermal stability. Anal Chem 69:3889–3898
Kumar A, Gaurav A, Malik K, Tewary DK, Singh B (2008) A review on development of solid phase microextraction fibers by sol-gel methods and their applications. Anal Chim Acta 610:1–14
Lee JW, Samal S, Selvapalam N, Kim HJ, Kim K (2003) Cucurbituril homologues and derivations: new opportunities in supramolecular chemistry. Acc Chem Res 36:621–630
Mock WL, Shin NY (1986) Structure and selectivity in host-guest complexes of cucurbituril. J Org Chem 51:4440–4446
Kim E, Kim D, Jung H, Lee J, Paul S, Selvapalam N, Yang Y, Lim N, Park CG, Kim K (2010) Facile, template-free synthesis of stimuli-responsive polymer nanocapsule for targeted drug delivery. Angew Chem Int Ed 49:4405–4408
Jeon WS, Ziganshina AY, Lee JW, Ko YH, Kang JK, Lee C, Kim K (2003) A[2]pseudorotaxane-based molecular machine: reversible formation of a molecular loop driven by electrochemical and photochemical stimuli. Angew Chem Int Ed 42:4097–4100
Dong N, Li T, Luo YJ, Shao L, Tao Z, Zhu C (2016) A solid-phase microextraction coating of sol-gel-derived perhydroxy cucurbit[6]uril and its application on to the determination of polycyclic aromatic hydrocarbon. J Chromatogr A 1470:9–18
Cong H, Ni XL, Xiao X, Huang Y, Zhu QJ, Xue SF, Tao Z, Lindoy LF, Wei G (2016) Synthesis and separation of cucurbit[n]urils and their derivatives. Org Biomol Chem 14:4335–4364
Ma D, Hettiarachchi G, Nguyen D, Zhang B, Wittenberg JB, Zavalij PY, Briken V, Isaacs L (2012) Acyclic curcubit[n]uril molecular contaniers enhance the solubility and bioactivity of poorly soluble pharmaceutical. Nat Chem 4:503–510
Ghale G, Ramalingam V, Urbach AR, Nau WM (2011) Determination protease substrate selectivity and inhibition by label-free supramolecular tandem enzyme assays. J Am Chem Soc 133:7528–7535
Jon SJ, Selvapalam N, Oh DH, Kang JK, Kim SY, Jeon YJ, Lee JW, Kim K (2003) Facil synthesis of cucurbit[n]uril derivatives via direct functionalization: expanding utilization of cucurbit[n]uril. J Am Chem Soc 125:10186–10187
Vinciguerra B, Cao L, Cannon JR, Zavalij PY, Fenselau C, Isaacs L (2012) Synthesis and self-assembly processes of monofunctionalized cucurbit[7]uril. Am Chem Soc 134:13133–13140
Zeng Z, Qiu W, Huang Z (2001) Solid-phase microextraction using fused-silica fibers coated with sol-gel-derived hydroxy-crown ether. Anal Chem 73:2429–2436
Dong N, He J, Li T, Peralta A, Avei MR, Ma MF, Kaifer AE (2018) Synthesis and binding properties of monohydroxycucurbit[7]uril: a key derivative for the functionalization of cucurbituril hosts. J Org Chem 83:5467–5473
Lei Y, Chen B, You L, He M, Hu B (2017) Polydimethylsiloxane/MIL-100(Fe) coated stir bar sorptive extraction-high performance liquid chromatography for the determination of triazines in environmental water samples. Talanta 175:158–167
Mourdikoudis S, Montes-García V, Rodal-Cedeira S, Winckelmans N, Pérez-Juste L, Wu H, Bals S, Pérez-Juste J, Patoriza-Santos I (2019) Highly porous palladium nanodenedrites: wet-chemical synthesis, electron tomography and catalytic activity. Dalton Trans 48:3758–3767
Shu J, Xie P, Lin D, Chen R, Wang J, Zhang B, Liu M, Liu H, Liu F (2014) Two highly stable and selective solid phase microextraction fibers coated with crown ether functionalized ionic liquids by different sol-gel reaction approaches. Anal Chim Acta 806:152–164
Zheng L (2018) Nanoporous silica-dye microspheres for enhanced colorimetric detection of cyclohexanoe. Chemosnesors 6:1–11
Zou X, Wang H, Lu Y, Huang C, Xia L, Chen X, Shen C, Chu Y (2015) Rapid analysis of residrual cyclohexanone in pvc infusion set by extractive electrospray ionization-mass spectrometer/mass spectrometer. Acta Chim Sin 73:851–855
Khalfi F, Dine T, Luyckx M, Gressier B, Brunet C, Ballester L, Cazin M, Cazin JC (1998) Determination of cyclohexanone after derivatization with 2,4- dinitrophenyl hydrazine in intravenous solution stored in pvc bags by high performace liquid chromatography. Biomed Chromatogr 12:69–72
Ni XN, Xiao X, Cong H, Zhu QJ, Xue SF, Tao Z (2014) Self-assemblies based on the “outer-surface interactions” of cucurbit[n]urils: new opportunities for supramoloecular architectures and materials. Acc Chem Res 47:1386–1395
Pinjari RV, Khedkar JK, Gejji SP (2010) Cavity diameter and height of cyclodextrins and cucurbit[n]urils from the molecular electrostatic potential topography. J Incl Phenom Macro 66:371–380
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NO.21665005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 24.5 mb)
Rights and permissions
About this article
Cite this article
Dong, N., Zhang, L., Yao, J. et al. Monohydroxycucurbit[7]uril-coated stir-bar sorptive extraction coupled with high-performance liquid chromatography for the determination of apolar and polar organic compounds. Microchim Acta 186, 846 (2019). https://doi.org/10.1007/s00604-019-3910-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3910-y