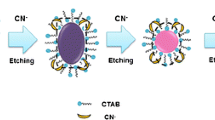
Abstract
A colorimetric and visual assay is described for the herbicide aminotriazole (ATZ). It is based on the etching of gold nanorods (AuNRs) by iodine which is formed on oxidation of iodide via H2O2. Longitudinal etching of the AuNRs occurs quickly and is accompanied by a color change from dark blue to red. In the absence of ATZ and the presence of active catalase (CAT), H2O2 is quickly decomposed into water, and the AuNRs will not be etched. In the presence of ATZ, CAT is partially deactivated, and this affects the amount of available H2O2 and, consequently, of the iodine. Hence, the color is significantly changed. The color changes can be easily detected with bare eyes. The assay has a linear response in the 5 to 70 μM concentration range, with a detection limit of 1.3 μM and high selectivity for ATZ. It was applied to the determination of ATZ in water and food samples.

A multicolor colorimetric method is developed for aminotriazole (ATZ) detection based on catalase (CAT) deactivation-dependent longitudinal etching of gold nanorods (AuNRs). The color signals can be visually identified. Good detection performances and capability for evaluating ATZ level in water and food samples is demonstrated.





Similar content being viewed by others
References
Catastini C, Rafqah S, Mailhot G, Sarakha M (2004) Degradation of amitrole by excitation of iron(III) aquacomplexes in aqueous solutions. J Photoch Photobio A 162:97–103
Da Pozzo A, Merli C, Sirés I, Garrido JA, Rodríguez RM, Brillas E (2005) Removal of the herbicide amitrole from water by anodic oxidation and electro-Fenton. Environ Chem Lett 3:7–11
Moreno M, Bermejo E, Sanchez A, Chicharro M, Zapardiel A (2008) Application of matrix solid-phase dispersion to the determination of amitrole and urazole residues in apples by capillary electrophoresis with electrochemical detection. Anal Bioanal Chem 391:867–872
Clark JM (1997) Insecticides as tools in probing vital receptors and enzymes in excitable membranes. Pestic Biochem Physiol 57:235–254
Bobeldijk I, Broess K, Speksnijder P, Leerdam TV (2001) Determination of the herbicide amitrole in water with pre-column derivatization, liquid chromatography and tandem mass spectrometry. J Chromatogr A 938:15–22
Maringa A, Mugadza T, Antunes E, Nyokong T (2013) Characterization and electrocatalytic behaviour of glassy carbon electrode modified with nickel nanoparticles towards amitrole detection. J Electroanal Chem 700:86–92
Sanchez-Bayo F, Hyne RV, Desseille KL (2010) An amperometric method for the detection of amitrole, glyphosate and its aminomethyl-phosphonic acid metabolite in environmental waters using passive samplers. Anal Chim Acta 675:125–131
Sun Y, Liu PF, Wang D, Li JQ, Cao YS (2009) Determination of amitrole in environmental water samples with precolumn derivatization by high-performance liquid chromatography. J Agric Food Chem 57:4540–4544
Pepich B, Brahmprakash DM, Dattilio T (2005) Development of U.S. EPA method 527 for the analysis of selected pesticides and flame retardants in the UCMR survey. Environ Sci Technol 39:4996–5004
Girod M, Delaurent C, Charles L (2006) Analysis of amitrole by normal-phase liquid chromatography and tandem mass spectrometry using a sheath liquid electrospray interface. Rapid Commun Mass Sp 20:892–896
Chicharro M, Moreno M, Bermejo E, Ongay S, Zapardiel A (2005) Determination of amitrole and urazole in water samples by capillary zone electrophoresis using simultaneous UV and amperometrical detection. J Chromatogr A 1099:191–197
Takeda S, Fukushi K, Chayama K, Nakayama Y, Tanaka Y, S-i W (2004) Simultaneous separation and on-line concentration of amitrole and benzimidazole pesticides by capillary electrophoresis with a volatile migration buffer applicable to mass spectrometric detection. J Chromatogr A 1051:297–301
Song Y, Wei W, Qu X (2011) Colorimetric biosensing using smart materials. Adv Mater 23:4215–4236
Tang L, Li J (2017) Plasmon-based colorimetric nanosensors for ultrasensitive molecular diagnostics. ACS Sens 2:857–875
Jiao YF, Liu QY, Qiang H, Chen ZB (2018) Colorimetric detection of L-histidine based on the target-triggered self-cleavage of swing-structured DNA duplex-induced aggregation of gold nanoparticles. Microchim Acta 185:452–458
Liu J, Lu Y (2006) Preparation of aptamer-linked gold nanoparticle purple aggregates for colorimetric sensing of analytes. Nat Protoc 1:246–252
Hu XL, Jin HY, He XP, James TD, Chen GR, Long YT (2015) Colorimetric and plasmonic detection of lectins using core-shell gold glyconanoparticles prepared by copper-free click chemistry. ACS Appl Mater Interfaces 7:1874–1878
Yeh YC, Creran B, Rotello VM (2012) Gold nanoparticles: preparation, properties, and applications in bionanotechnology. Nanoscale 4:1871–1880
Chen H, Shao L, Li Q, Wang J (2013) Gold nanorods and their plasmonic properties. Chem Soc Rev 42:2679–2724
Howes PD, Rana S, Stevens MM (2014) Plasmonic nanomaterials for biodiagnostics. Chem Soc Rev 43:3835–3853
Li J, Deng T, Chu X, Yang RH, Jiang J, Shen G, Yu R (2010) Rolling circle amplification combined with gold nanoparticle aggregates for highly sensitive identification of single-nucleotide polymorphisms. Anal Chem 82:2811–2816
Wang H, Wang Y, Jin J, Yang R (2008) Gold nanoparticle-based colorimetric and " turn-on " fluorescent probe for mercury(II) ions in aqueous solution. Anal Chem 80:9021–9028
Liu QL, Li L, Zhao Y, Chen ZB (2018) Colorimetric detection of DNA at the nanomolar level based on enzyme-induced gold nanoparticle de-aggregation. Microchim Acta 185:301–307
Li F, Feng Y, Zhao C, Tang B (2011) Simple colorimetric sensing of trace bleomycin using unmodified gold nanoparticles. Biosens Bioelectron 26:4628–4631
Zhou ML, Lin T, Gan XX (2017) Colorimetric aggregation assay for silver(I) based on the use of aptamer modified gold nanoparticles and C-ag(I)-C interaction. Microchim Acta 184:4671–4677
Chen Z, Chen C, Huang H, Luo F, Guo L, Zhang L, Lin Z, Chen G (2018) Target-induced horseradish peroxidase deactivation for multicolor colorimetric assay of hydrogen sulfide in rat brain microdialysis. Anal Chem 90:6222–6228
Wu S, Li DD, Gao ZZ, Wang JM (2017) Controlled etching of gold nanorods by the au(III)-CTAB complex, and its application to semi-quantitative visual determination of organophosphorus pesticides. Microchim Acta 184:4383–4391
Zhao Q, Huang H, Zhang L, Wang L, Zeng Y, Xia X, Liu F, Chen Y (2016) Strategy to fabricate naked-eye readout ultrasensitive Plasmonic Nanosensor based on enzyme mimetic gold nanoclusters. Anal Chem 88:1412–1418
Ma X, Lin Y, Guo L, Qiu B, Chen G, Yang HH, Lin Z (2017) A universal multicolor immunosensor for semiquantitative visual detection of biomarkers with the naked eyes. Biosens Bioelectron 87:122–128
Nicholls P (1962) The reaction between Aminotriazole and catalase. Biochim Biophys Acta 59:414–420
Davis A, Tran T, Young DR (1993) Solution chemistry of iodide leaching of gold. Hydrometallurgy 32:143–159
Nikoobakht B, El-Sayed MA (2003) Preparation and growth mechanism of gold Nanorods(NRs) using seed-mediated growth method. Chem Mater 15:1957–1962
Zhang Z, Chen Z, Chen L (2015) Ultrasensitive visual sensing of Molybdate based on enzymatic-like etching of gold Nanorods. Langmuir 31:9253–9259
Huang X, Neretina S, El-Sayed MA (2009) Gold nanorods: from synthesis and properties to biological and biomedical applications. Adv Mater 21:4880–4910
Analytical Methods Committee (1987) Recommendations for the definition, estimation and use of the detection limit. Analyst 112:199–204
Acknowledgements
This work was supported in part by the financial support through the National Natural Science Foundation of China (21605008, 21575018, 21735001, 21705010), and the Natural Science Foundation of Hunan Province (2019JJ30025).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 1178 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Luo, G., Qing, Z. et al. Colorimetric aminotriazole assay based on catalase deactivation-dependent longitudinal etching of gold nanorods. Microchim Acta 186, 565 (2019). https://doi.org/10.1007/s00604-019-3677-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3677-1