Abstract
Graphene oxide was covalently modified with p-phenylenediamine via a diazonium reaction. The resulting material was employed as a sorbent for the solid-phase extraction of six phenylurea herbicides (metoxuron, monuron, chlortoluron, isoproturon, monolinuron, and buturon) from environmental water and lettuce leafs. Some key factors that influence the extraction efficiency were studied, including sample loading rate, sample pH, and desorption conditions. Following desorption with acetonitrile, the analytes were quantified by HPLC with UV detection. Under optimized conditions, response to phenylurea herbicides is linear in the 2.0–100 ng mL−1 concentration range for water samples, and 5.0–100 ng g−1 for leaf lettuces. The limits of detection are 0.10–0.25 ng mL−1 for water samples, and 1.5–2.5 ng g−1 for leaf lettuces. The sorbent was also applied to the preconcentration of organic compounds including nitroimidazoles, chlorophenols, phenylurea insecticides and phthalates. This shows that this sorbent has a large potential for the enrichment of organic pollutants.

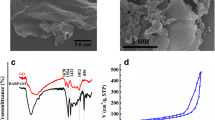
A graphene oxide/p-phenylenediamine (GO@PA) composite was prepared via a simple and environmentally-friendly diazonium reaction between p-phenylenediamine diazonium salt and graphene oxide. It was used as the solid phase extraction (SPE) adsorbent to extract phenylurea herbicides with the extraction efficiency higher than that of commercial C18, multi-walled carbon nanotube and polystyrene polymer. The SPE method was combined with HPLC for simultaneous determination of six phenylurea herbicides in environment water and vegetable samples.



Similar content being viewed by others
References
Farajzadeh MA, Mohebbi A, Feriduni B (2016) Development of continuous dispersive liquid-liquid microextraction performed in home-made device for extraction and preconcentration of aryloxyphenoxy-propionate herbicides from aqueous samples followed by gas chromatography-flame ionization detection. Anal Chim Acta 920:1–9. https://doi.org/10.1016/j.aca.2016.03.041
Nélieu S, Bonnemoy F, Bonnet JL, Lefeuvre L, Baudiffier D, Heydorff M, Quéméneur A, Azam D, Ducrot PH, Lagadic L, Bohatier J, Einhorn J (2010) Ecotoxicological effects of diuron and chlorotoluron nitrate-induced photodegradation products: monospecific and aquatic mesocosm-integrated studies. Environ Toxicol Chem 29:2644–2652. https://doi.org/10.1002/etc.341
Baskeyfield DEH, Davis F, Magan N, Tothill IE (2011) A membrane-based immunosensor for the analysis of the herbicide isoproturon. Anal Chim Acta 699:223–231. https://doi.org/10.1016/j.aca.2011.05.036
Sapozhnikova Y, Wirth E, Singhasemanon N, Bacey J, Fulton M (2008) Distribution of antifouling biocides in California marinas. J Environ Monit 10:1069–1075. https://doi.org/10.1039/B806934D
Gatidou G, Thomaidis NS, Zhou JL (2007) Fate of Irgarol 1051, diuron and their main metabolites in two UK marine systems after restrictions in antifouling paints. Environ Int 33:70–77. https://doi.org/10.1016/j.envint.2006.07.002
Abbot J, Marohasy J (2011) Has the herbicide Diuron caused mangrove dieback? A re-examination of the evidence. Hum Ecol Risk Assess 17:1077–1094. https://doi.org/10.1080/10807039.2011.605672
de Lima F, Gozzi F, Fiorucci AR, Cardoso CAL, Arruda GJ, Ferreira VS (2011) Determination of linuron in water and vegetable samples using stripping voltammetry with a carbon paste electrode. Talanta 83:1763–1768. https://doi.org/10.1016/j.talanta.2010.12.014
Liu X, Wang C, Wu Q, Wang Z (2015) Metal-organic framework-templated synthesis of magnetic nanoporous carbon as an efficient absorbent for enrichment of phenylurea herbicides. Anal Chim Acta 870:67–74. https://doi.org/10.1016/j.aca.2015.02.036
Su M, Jia L, Wu X, Sun H (2018) Residue investigation of some phenylureas and tebuthiuron herbicides in vegetables by ultra-performance liquid chromatography coupled with integrated selective accelerated solvent extraction-clean up in situ. J Sci Food Agric 98:4845–4853. https://doi.org/10.1002/jsfa.9014
Escuderos-Morenas ML, Santos-Delgado MJ, Rubio-Barroso S, Polo-Dı́ez LM (2003) Direct determination of monolinuron, linuron and chlorbromuron residues in potato samples by gas chromatography with nitrogen–phosphorus detection. J Chromatogr A 1011:143–153. https://doi.org/10.1016/S0021-9673(03)01139-7
Rodriguez-Mozaz S, Lopez de Alda MJ, Barceló D (2007) Advantages and limitations of on-line solid phase extraction coupled to liquid chromatography–mass spectrometry technologies versus biosensors for monitoring of emerging contaminants in water. J Chromatogr A 1152:97–115. https://doi.org/10.1016/j.chroma.2007.01.046
Adelhelm C, Niessner R, Poschl U, Letzel T (2008) Analysis of large oxygenated and nitrated polycyclic aromatic hydrocarbons formed under simulated diesel engine exhaust conditions (by compound fingerprints with SPE/LC-API-MS). Anal Bioanal Chem 391:2599–2608. https://doi.org/10.1007/s00216-008-2175-9
Płotka-Wasylka J, Szczepańska N, De la Guardia M, Namieśnik J (2016) Modern trends in solid phase extraction: new sorbent media. Trends Anal Chem 77:23–43. https://doi.org/10.1016/j.trac.2015.10.010
Jiménez-Soto JM, Cárdenas S, Valcárcel M (2009) Evaluation of carbon nanocones/disks as sorbent material for solid-phase extraction. J Chromatogr A 1216:5626–5633. https://doi.org/10.1016/j.chroma.2009.05.070
Muñoz J, Gallego M, Valcárcel M (2004) Solid-phase extraction–gas chromatography–mass spectrometry using a fullerene sorbent for the determination of inorganic mercury(II), methylmercury(I) and ethylmercury(I) in surface waters at sub-ng/ml levels. J Chromatogr A 1055:185–190. https://doi.org/10.1016/j.chroma.2004.09.026
Savio M, Parodi B, Martinez LD, Smichowski P, Gil RA (2011) On-line solid phase extraction of Ni and Pb using carbon nanotubes and modified carbon nanotubes coupled to ETAAS. Talanta 85:245–251. https://doi.org/10.1016/j.talanta.2011.03.054
Wang Y, Gao S, Zang X, Li J, Ma J (2012) Graphene-based solid-phase extraction combined with flame atomic absorption spectrometry for a sensitive determination of trace amounts of lead in environmental water and vegetable samples. Anal Chim Acta 716:112–118. https://doi.org/10.1016/j.aca.2011.12.007
Wu Q, Wang C, Liu Z, Wu C, Zeng X, Wen J, Wang Z (2009) Dispersive solid-phase extraction followed by dispersive liquid-liquid microextraction for the determination of some sulfonylurea herbicides in soil by high-performance liquid chromatography. J Chromatogr A 1216:5504–5510. https://doi.org/10.1016/j.chroma.2009.05.062
Singh V, Joung D, Zhai L, Das S, Khondaker SI, Seal S (2011) Graphene based materials: past, present and future. Prog Mater Sci 56:1178–1271. https://doi.org/10.1016/j.pmatsci.2011.03.003
Sitko R, Zawisza B, Malicka E (2013) Graphene as a new sorbent in analytical chemistry. Trac-Trend Anal Chem 51:33–43. https://doi.org/10.1016/j.trac.2013.05.011
Reddy KR, Sin BC, Ryu KS, Kim J-C, Chung H, Lee Y (2009) Conducting polymer functionalized multi-walled carbon nanotubes with noble metal nanoparticles: synthesis, morphological characteristics and electrical properties. Synth Met 159:595–603. https://doi.org/10.1016/j.synthmet.2008.11.030
Peng W, Li H, Liu Y, Song S (2017) A review on heavy metal ions adsorption from water by graphene oxide and its composites. J Mol Liq 230:496–504. https://doi.org/10.1016/j.molliq.2017.01.064
Lee J, Kim J, Kim S, Min D-H (2016) Biosensors based on graphene oxide and its biomedical application. Adv Drug Deliv Rev 105:275–287. https://doi.org/10.1016/j.addr.2016.06.001
Zhao G, Ren X, Gao X, Tan X, Li J, Chen C, Huang Y, Wang X (2011) Removal of Pb(II) ions from aqueous solutions on few-layered graphene oxide nanosheets. Dalton Trans 40:10945–10952. https://doi.org/10.1039/c1dt11005e
Sun Y, Wang Q, Chen C, Tan X, Wang X (2012) Interaction between Eu(III) and graphene oxide nanosheets investigated by batch and extended X-ray absorption fine structure spectroscopy and by modeling techniques. Environ Sci Technol 46:6020–6027. https://doi.org/10.1021/es300720f
Ghanem A, Adly FG, Sokerik Y, Antwi NY, Shenashen MA, El-Safty SA (2017) Trimethyl- β -cyclodextrin-encapsulated monolithic capillary columns: preparation, characterization and chiral nano-LC application. Talanta 169:239–248. https://doi.org/10.1016/j.talanta.2016.06.027
Compton OC, Dikin DA, Putz KW, Brinson LC, Nguyen ST (2010) Electrically conductive "alkylated" graphene paper via chemical reduction of amine-functionalized graphene oxide paper. Adv Mater 22:892–896. https://doi.org/10.1002/adma.200902069
Fang F, Kong L, Huang J, Wu S, Zhang K, Wang X, Sun B, Jin Z, Wang J, Huang XJ, Liu J (2014) Removal of cobalt ions from aqueous solution by an amination graphene oxide nanocomposite. J Hazard Mater 270:1–10. https://doi.org/10.1016/j.jhazmat.2014.01.031
Sun M, Tang R, Wu Q, Wang C, Wang Z (2013) Graphene reinforced hollow fiber liquid-phase microextraction for the determination of phthalates in water, juice and milk samples by HPLC. Anal Methods-UK 5:5694. https://doi.org/10.1039/c3ay40966j
Wang W, Ma R, Wu Q, Wang C, Wang Z (2013) Fabrication of magnetic microsphere-confined graphene for the preconcentration of some phthalate esters from environmental water and soybean milk samples followed by their determination by HPLC. Talanta 109:133–140. https://doi.org/10.1016/j.talanta.2013.02.008
De la Peña AM, Mahedero MC, Bautista-Sánchez A (2003) Monitoring of phenylurea and propanil herbicides in river water by solid-phase-extraction high performance liquid chromatography with photoinduced-fluorimetric detection. Talanta 60:279–285. https://doi.org/10.1016/S0039-9140(03)00072-9
Lin H-H, Sung Y-H, Huang S-D (2003) Solid-phase microextraction coupled with high-performance liquid chromatography for the determination of phenylurea herbicides in aqueous samples. J Chromatogr A 1012:57–66. https://doi.org/10.1016/S0021-9673(03)01169-5
Gao S, You J, Zheng X, Wang Y, Ren R, Zhang R, Bai Y, Zhang H (2010) Determination of phenylurea and triazine herbicides in milk by microwave assisted ionic liquid microextraction high-performance liquid chromatography. Talanta 82:1371–1377. https://doi.org/10.1016/j.talanta.2010.07.002
Chou T-Y, Lin S-L, Fuh M-R (2009) Determination of phenylurea herbicides in aqueous samples using partitioned dispersive liquid–liquid microextraction. Talanta 80:493–498. https://doi.org/10.1016/j.talanta.2009.07.005
Acknowledgements
Financial supports from the National Natural Science Foundation of China (31571925, 31671930), the Natural Science Foundation of Hebei Province (B2016204136, B2016204146, B2017204025, C2018204076), Preferential Foundation for the Introduction of Overseas Scholars by Hebei Province (CL201713), the Natural Science Foundation of Hebei Agricultural University (LG201607, LG201610, ZD201703, LG201712) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human or animal subjects.
Informed consent
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 2340 kb)
Rights and permissions
About this article
Cite this article
Guo, L., Hao, L., Gao, T. et al. p-Phenylenediamine-modified graphene oxide as a sorbent for solid-phase extraction of phenylurea herbicides, nitroimidazoles, chlorophenols, phenylurea insecticides and phthalates. Microchim Acta 186, 464 (2019). https://doi.org/10.1007/s00604-019-3606-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3606-3