Abstract
It is found that catechol inhibits the oxidase-mimicking activity of chitosan-protected platinum nanoparticles (Chit-PtNPs) by competing with the substrate for the active site of the Ch-PtNPs. The inhibition mechanism of catechol is different from that of ascorbic acid in that it neither reacts with O2•- nor reduces the oxidized 3,3′,5,5′-tetramethylbenzidine (TMB). Tyrosinase (TYRase) catalyzes the oxidation of catechol, thus restoring the activity of oxidase-mimicking Chit-PtNPs. By combining the Chit-PtNP, catechol, and TYRase interactions with the oxidation of TMB to form a yellow diamine (maximal absorbance at 450 nm), a colorimetric analytical method was developed for TYRase determination and inhibitor screening. The assay works in the 0.5 to 2.5 U·mL−1 TYRase activity range, and the limit of detection is 0.5 U·mL−1. In our perception, this new assay represents a powerful approach for determination of TYRase activity in biological samples.

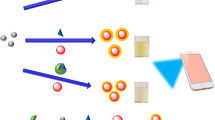
Schematic representation of a colorimetric method for tyrosinase (TYRase) detection and inhibitor screening. It is based on the fact that catechol can inhibit the oxidase-like activity of chitosan-stabilized platinum nanoparticles (Ch-PtNPs) by competing with the substrate for the active sites and TYRase can catalyze the oxidation of catechol.







Similar content being viewed by others
References
Ragg R, Natalio F, Tahir MN, Janssen H, Kashyap A, Strand D, Strand S, Tremel W (2014) Molybdenum trioxide nanoparticles with intrinsic sulfite oxidase activity. ACS Nano 8(5):5182–5189
Lang NJ, Liu BW, Liu JW (2014) Characterization of glucose oxidation by gold nanoparticles using nanoceria. J Colloid Interface Sci 428:78–83
He XL, Tan LF, Chen D, Wu XL, Ren XL, Zhang YQ, Meng XW, Tang FQ (2013) Fe3O4-au@mesoporous SiO2 microspheres: an ideal artificial enzymatic cascade system. Chem Commun 49(41):4643–4645
Natalio F, Andre R, Hartog AF, Stoll B, Jochum KP, Wever R, Tremel W (2012) Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat Nanotechnol 7(8):530–535
Gao LZ, Zhuang J, Nie L, Zhang JB, Zhang Y, Gu N, Wang TH, Feng J, Yang DL, Perrett S, Yan X (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2(9):577–583
Wei H, Wang E (2008) Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal Chem 80(6):2250–2254
Deng HH, Hong GL, Lin FL, Liu AL, Xia XH, Chen W (2016) Colorimetric detection of urea, urease, and urease inhibitor based on the peroxidase-like activity of gold nanoparticles. Anal Chim Acta 915:74–80
Hong L, Liu AL, Li GW, Chen W, Lin XH (2013) Chemiluminescent cholesterol sensor based on peroxidase-like activity of cupric oxide nanoparticles. Biosens Bioelectron 43:1–5
Peng HP, Lin DW, Liu P, Wu YH, Li SH, Lei Y, Chen W, Chen YZ, Lin XH, Xia XH, Liu AL (2017) Highly sensitive and rapid colorimetric sensing platform based on water-soluble WOx quantum dots with intrinsic peroxidase-like activity. Anal Chim Acta 992:128–134
Li YZ, Li TT, Chen W, Song YY (2017) Co4N nanowires: Noble-metal-free peroxidase mimetic with excellent salt- and temperature-resistant abilities. ACS Appl Mater Interfaces 9(35):29881–29888
Zhuang QQ, Lin ZH, Jiang YC, Deng HH, He SB, Su LT, Shi XQ, Chen W (2017) Peroxidase-like activity of nanocrystalline cobalt selenide and its application for uric acid detection. Int J Nanomedicine 12:3295–3302
Cai SF, Qi C, Li YD, Han QS, Yang R, Wang C (2016) PtCo bimetallic nanoparticles with high oxidase-like catalytic activity and their applications for magnetic-enhanced colorimetric biosensing. J Mater Chem B 4(10):1869–1877
Xu C, Qu XG (2014) Cerium oxide nanoparticle: a remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater 6:e90
Wang GL, Jin LY, Wu XM, Dong YM, Li ZJ (2015) Label-free colorimetric sensor for mercury(II) and DNA on the basis of mercury(II) switched-on the oxidase-mimicking activity of silver nanoclusters. Anal Chim Acta 871:1–8
Asati A, Kaittanis C, Santra S, Perez JM (2011) pH-tunable oxidase-like activity of cerium oxide nanoparticles achieving sensitive fluorigenic detection of cancer biomarkers at neutral pH. Anal Chem 83(7):2547–2553
Pal J, Pal T (2016) Enzyme mimicking inorganic hybrid Ni@MnO2 for colorimetric detection of uric acid in serum samples. RSC Adv 6(87):83738–83747
Zhang SX, Xue SF, Deng JJ, Zhang M, Shi GY, Zhou TS (2016) Polyacrylic acid-coated cerium oxide nanoparticles: an oxidase mimic applied for colorimetric assay to organophosphorus pesticides. Biosens Bioelectron 85:457–463
Pautler R, Kelly EY, Huang P-JJ, Cao J, Liu B, Liu J (2013) Attaching DNA to nanoceria: regulating oxidase activity and fluorescence quenching. ACS Appl Mater Interfaces 5(15):6820–6825
Liu J, Hu X, Hou S, Wen T, Liu W, Zhu X, Wu X (2011) Screening of inhibitors for oxidase mimics of au@Pt nanorods by catalytic oxidation of OPD. Chem Commun 47(39):10981–10983
Guo R, Wang Y, Yu S, Zhu W, Zheng F, Liu W, Zhang D, Wang J (2016) Dual role of hydrogen peroxide on the oxidase-like activity of nanoceria and its application for colorimetric hydrogen peroxide and glucose sensing. RSC Adv 6(65):59939–59945
Deng HH, Lin XL, Liu YH, Li KL, Zhuang QQ, Peng HP, Liu AL, Xia XH, Chen W (2017) Chitosan-stabilized platinum nanoparticles as effective oxidase mimics for colorimetric detection of acid phosphatase. Nanoscale 9(29):10292–10300
Briganti S, Camera E, Picardo M (2003) Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res 16(2):101–110
Bai M, Huang J, Zheng X, Song Z, Tang M, Mao W, Yuan L, Wu J, Weng X, Zhou X (2010) Highly selective suppression of melanoma cells by inducible DNA cross-linking agents: Bis(catechol) derivatives. J Am Chem Soc 132(43):15321–15327
Sidhu JS, Singh N (2018) FRET and PET paired dual mechanistic carbon dots approach for TYRaseosinase sensing. J Mater Chem B 6(24):4139–4145
Peng M, Wang Y, Fu Q, Sun F, Na N, Ouyang J (2018) Melanosome-targeting near-infrared fluorescent probe with large stokes shift for in situ quantification of TYRaseosinase activity and assessing drug effects on differently invasive melanoma cells. Anal Chem 90(10):6206–6213
Lei C, Zhao X, Sun J, Yan X, Gao Y, Gao H, Zhu S, Wang H (2017) A simple and novel colorimetric assay for tyrosinase and inhibitor screening using 3,3′,5,5′-tetramethylbenzidine as a chromogenic probe. Talanta 175:457–462
Freeman R, Elbaz J, Gill R, Zayats M, Willner I (2007) Analysis of dopamine and tyrosinase activity on ion-sensitive field-effect transistor (ISFET) devices. Chem A Eur J 13(26):7288–7293
Teng Y, Jia X, Li J, Wang E (2015) Ratiometric fluorescence detection of tyrosinase activity and dopamine using thiolate-protected gold nanoclusters. Anal Chem 87(9):4897–4902
Yang X, Luo Y, Zhuo Y, Feng Y, Zhu S (2014) Novel synthesis of gold nanoclusters templated with L-tyrosine for selective analyzing tyrosinase. Anal Chim Acta 840:87–92
Wu X, Li L, Shi W, Gong Q, Ma H (2016) Near-infrared fluorescent probe with new recognition moiety for specific detection of tyrosinase activity: design, synthesis, and application in living cells and zebrafish. Angew Chem Int Ed 55(47):14728–14732
Li H, Liu W, Zhang F, Zhu X, Huang L, Zhang H (2017) Highly selective fluorescent probe based on hydroxylation of phenylboronic acid pinacol ester for detection of tyrosinase in cells. Anal Chem 90(1):855–858
Kong F, Liu H, Dong J, Qian W (2011) Growth-sensitive gold nanoshells precursor nanocomposites for the detection of L-DOPA and tyrosinase activity. Biosens Bioelectron 26(5):1902–1907
Liu B, Huang P, Li J, Wu F (2017) Colorimetric detection of tyrosinase during the synthesis of kojic acid/silver nanoparticles under illumination. Sens Actuators B Chem 251:836–841
Baek H, Rho H, Yoo J, Ahn S, Lee J, Lee J, Kim M, Kim D, Chang I (2008) The inhibitory effect of new hydroxamic acid derivatives on melanogenesis. B Korean Chem Soc 29(1):43–46
Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (21675024, 21804021), the Program for Innovative Leading Talents in Fujian Province (2016B016), the Science and Technology Project of Fujian Province (2018 L3008), Natural Science Foundation of Fujian Province (2016 J01427, 2016 J06019), and Startup Fund for scientific research, Fujian Medical University (2017XQ1014).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 236 kb)
Rights and permissions
About this article
Cite this article
Deng, HH., Lin, XL., He, SB. et al. Colorimetric tyrosinase assay based on catechol inhibition of the oxidase-mimicking activity of chitosan-stabilized platinum nanoparticles. Microchim Acta 186, 301 (2019). https://doi.org/10.1007/s00604-019-3451-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3451-4