Abstract
Gold nanoparticles (AuNPs) catalyze the mild reaction between the weak reducing agent kojic acid (KA) and silver ions (Ag+) to form Au@Ag bimetallic NPs by the combination of the intrinsic catalysis with plasmonic properties This is proposed as a novel optical assay to determine the tyrosinase (TYRase) concentration. The nanoparticles have been characterized by UV-vis spectroscopy, transmission electron microscope (TEM) images, and X-ray photoelectron spectroscopy (XPS). The sensing mechanism is based on the fact that KA binds to TYRase by chelating with dicopper active site of TYRase and the introduction of TYRase restrains the Au@Ag bimetallic NP formation by the precedent binding with KA. A clear color variation from yellow to pink and UV-vis spectral changes are observed at the optimal wavelength of 410 nm. The assay works in the range 0.13~0.73 U mL−1 with a detection limit (LOD) of 0.019 U mL−1. The impact from matrix interfering substances including glucose, uric acid, common oxidases, and amino acids is negligible. The applicability is demonstrated by quantitative determination of TYRase in human serum samples with 74 to 89% recovery and RSD less than 4.0%, which accords with the level for bio-sample analysis.

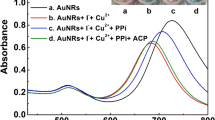
Schematic presentation of colorimetric assay for tyrosinase (TYRase) based on the inhibition effect on silver deposition onto catalytically active gold nanoparticles (AuNPs) and its application with a smartphone. Tyrosinase (TYRase); silver ions (Ag+); kojic acid (KA); gold nanoparticles (AuNPs); gold-silver core-shell nanoparticles (Au@Ag NPs)






Similar content being viewed by others
References
Huang XM, Zhao HN, Qiu W, Wang J, Guo LH, Lin ZY, Pan W, Wu Y, Qiu B (2020) A fluorescence signal amplification strategy for modification-free ratiometric determination of tyrosinase in situ based on the use of dual-templated copper nanoclusters. Microchim Acta 187:240–249. https://doi.org/10.1007/s00604-020-4186-y
Ramsden CA, Riley PA (2014) Tyrosinase: the four oxidation states of the active site and their relevance to enzymatic activation, oxidation and inactivation. Bioorg Med Chem 22:2388–2395. https://doi.org/10.1016/j.bmc.2014.02.048
Rolff M, Schottenheim J, Decker H, Tuczek F (2011) Copper–O2 reactivity of tyrosinase models towards external monophenolic substrates: molecular mechanism and comparison with the enzyme. Chem Soc Rev 40:4077–4098. https://doi.org/10.1039/C0CS00202J
Tessari I, Bisaglia M, Velle F, Samori B, Bergantino E, Mammi S, Bubacco L (2008) The reaction of α-synuclein with tyrosinase: possible implications for Parkinson disease. J Biol Chem 283:16808–16817. https://doi.org/10.1074/jbc.M709014200
Hasegawa T, Treis A, Patenge N, Fiesel FC, Springer W, Kahle PJ (2008) Parkin protects against tyrosinase-mediated dopamine neurotoxicity by suppressing stress-activated protein kinase pathways. J Neurochem 105:1700–1715. https://doi.org/10.1111/j.1471-4159.2008.05277.x
Vargas AJ, Sittadjody S, Thangasamy T, Mendoza E, Limesand KH, Burd R (2011) Exploiting tyrosinase expression and activity in melanocytic tumors: quercetin and the central role of p53. Integr Cancer Ther 10:328–340. https://doi.org/10.1177/2F1534735410391661
Zhao XE, Zuo YN, Qu XQ, Sun J, Liu LY, Zhu SY (2019) Colorimetric determination of the activities of tyrosinase and catalase via substrate-triggered decomposition of MnO2 nanosheets. Microchim Acta 186:848. https://doi.org/10.1007/s00604-019-3995-3
Xu QL, Yoon JY (2011) Visual detection of dopamine and monitoring tyrosinase activity using a pyrocatechol violet–Sn4+ complex. Chem Commun 47:12497–12499. https://doi.org/10.1039/C1CC15587C
Deng HH, Lin XL, He SB, Wu GW, Wu WH, Yang Y (2019) Colorimetric tyrosinase assay based on catechol inhibition of the oxidase-mimicking activity of chitosan-stabilized platinum nanoparticles. Microchim Acta 186:301. https://doi.org/10.1007/s00604-019-3451-4
Qu ZY, Yu T, Bi LH (2019) A dual-channel ratiometric fluorescent probe for determination of the activity of tyrosinase using nitrogen-doped graphene quantum dots and dopamine-modified Cd-Te quantum dots. Microchim Acta 186:635. https://doi.org/10.1007/s00604-019-3733-x
Yang XM, Luo YW, Zhuo Y, Feng YJ, Zhu SS (2014) Novel synthesis of gold nanoclusters templated with L-tyrosine for selective analyzing tyrosinase. Anal Chim Acta 840:87–92. https://doi.org/10.1016/jaca.2014.05.050
Ma XG, Gao WY, Halawa MI, Lan YX, Li JP, Xu GB (2019) Lucigenin fluorescent assay of Tyrosinase activity and its inhibitor screening. Sensors Actuators B Chem 280:41–45. https://doi.org/10.1016/j.snb.2018.10.044
Wang L, Gan ZF, Guo D, Xia HL, Patrice FT, Hafez ME (2019) Electrochemistry-regulated recyclable SERS sensor for sensitive and selective detection of tyrosinase activity. Anal Chem 91:6507–6513. https://doi.org/10.1021/acs.analchem.8b05341
Yildiz HB, Freeman R, Gill R, Willner I (2008) Electrochemical, photoelectrochemical, and piezoelectric analysis of tyrosinase activity by functionalized nanoparticles. Anal Chem 80:2811–2816. https://doi.org/10.1021/ac702401v
Xia N, Liu L, Chang Y, Hao YQ, Wang XJ (2017) 4-Mercaptophenyl-boronic acid-induced in situ formation of silver nanoparticle aggregates as labels on an electrode surface. Electrochem Commun 74:28–32. https://doi.org/10.1016/j.elecom.2016.11.013
Tang CC, Jin L, Lin YX, Su J, Sun YG, Liu P (2018) Aminoluciferin 4-hydroxyphenyl amide enables bioluminescence detection of endogenous tyrosinase. Org Biomol Chem 16:9197–9203. https://doi.org/10.1039/C8OB01777H
Song YJ, Wei WL, Qu XG (2011) Colorimetric biosensing using smart materials. Adv Mater 23:4215–4236. https://doi.org/10.1002/adma.201101853
Ma XM, He S, Qiu B, Luo F, Guo LH, Lin ZY (2019) Noble metal nanoparticle-based multicolor immunoassays: an approach toward visual quantification of the analytes with the naked eye. ACS Sens 4:782–791. https://doi.org/10.1021/acssensors.9b00438
Li JJ, Xi HY, Kong CY, Liu QY, Chen ZB (2018) “Aggregation-to-deaggregation” colorimetric signal amplification strategy for Ag+ detection at the femtomolar level with dark-field microscope observation. Anal Chem 90:11723–11727. https://doi.org/10.1021/acs.analchem.8b03739
Peng MP, Ma W, Long YT (2015) Alcohol dehydrogenase-catalyzed gold nanoparticle seed-mediated growth allows reliable detection of disease biomarkers with the naked eye. Anal Chem 87:5891–5896. https://doi.org/10.1021/acs.analchem.5b00287
Chen CX, Zhang GL, Ni PJ, Jiang YY, Lu YZ, Lu ZL (2019) Fluorometric and colorimetric dual-readout alkaline phosphatase activity assay based on enzymatically induced formation of colored Au@Ag nanoparticles and an inner filter effect. Microchim Acta 186:348. https://doi.org/10.1007/s00604-019-3478-6
Lv BJ, Wei M, Liu YJ, Liu X, Wei W, Liu SQ (2016) Ultrasensitive photometric and visual determination of organophosphorus pesticides based on the inhibition of enzyme-triggered formation of core-shell gold-silver nanoparticles. Microchim Acta 183:2941–2948. https://doi.org/10.1007/s00604-016-1939-8
Xu SH, Ouyang WJ, Xie PS, Lin Y, Qiu B, Lin ZY (2017) Highly uniform gold nanobipyramids for ultrasensitive colorimetric detection of influenza virus. Anal Chem 89:1617–1623. https://doi.org/10.1021/acs.analchem.6b03711
Liu YH, Pan M, Wang WX, Jiang QY, Wang FA, Pang DW (2019) Plasmonic and photothermal immunoassay via enzyme-triggered crystal growth on gold nanostars. Anal Chem 91:2086–2092. https://doi.org/10.1021/acs.analchem.8b04517
Donati P, Moglianetti M, Veronesi M, Prato M, Tatulli G, Bandiera T, Pompa PP (2019) Nanocatalyst/nanoplasmon-enabled detection of organic mercury: a one-minute visual test. Angew Chem Int Ed 58:10285–10289. https://doi.org/10.1002/anie.201905669
Faig J, Moretti A, Joseph L, Zhang YY, Nova MJ, Smith K (2017) Biodegradable kojic acid-based polymers: controlled delivery of bioactives for melanogenesis inhibition. Biomacromolecules 18:363–373. https://doi.org/10.1021/acs.biomac.6b01353
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci 241:20–22. https://doi.org/10.1038/physci241020a0
Liu BW, Huang PC, Li JF, Wu FY (2017) Colorimetric detection of tyrosinase during the synthesis of kojic acid/silver nanoparticles under illumination. Sensors Actuators B Chem 251:836–841. https://doi.org/10.1016/j.snb.2017.05.129
Ao H, Qian ZS, Zhu YY, Zhao MZ, Tang C, Huang YY (2016) A fluorometric biosensor based on functional Au/Ag nanoclusters for real-time monitoring of tyrosinase activity. Biosens Bioelectron 86:542–547. https://doi.org/10.1016/j.bios.2016.07.051
Li HH, Liu W, Zhang FY, Zhu XY, Huang LQ, Zhang HX (2018) Highly selective fluorescent probe based on hydroxylation of phenylboronic acid pinacol ester for detection of tyrosinase in cells. Anal Chem 90:855–858. https://doi.org/10.1021/acs.analchem.7b03681
Lei CH, Zhao XN, Sun J, Yan XL, Gao Y, Gao H (2017) A simple and novel colorimetric assay for tyrosinase and inhibitor screening using 3,3′,5,5′-tetramethyl-benzidine as a chromogenic probe. Talanta 175:457–462. https://doi.org/10.1016/j.talanta.2017.07.070
Durmazel S, Uzer A, Erbil B, Sayın B, Apak R (2019) Silver nanoparticle formation-based colorimetric determination of reducing sugars in food extracts via Tollens’ reagent. ACS Omega 4:7596–7604. https://doi.org/10.1021/acsomega.9b00761
Nishioka K, Romsdahl MM, Fritsche HA Jr, Mcmurtr MJ (1977) Tyrosinase activity in the sera of patients with malignant melanoma: method and specificity. Int J Cancer 20:689–693. https://doi.org/10.1002/ijc.2910200507
Funding
This work was financially supported by National Natural Science Foundation of China (Nos. 21765014 and 21864018) and the Science and Technology Innovation Platform Project of Jiangxi Province (No. 20192BCD40001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1062 kb)
Rights and permissions
About this article
Cite this article
Liu, H., Liu, B., Huang, P. et al. Colorimetric determination of tyrosinase based on in situ silver metallization catalyzed by gold nanoparticles. Microchim Acta 187, 551 (2020). https://doi.org/10.1007/s00604-020-04463-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04463-9