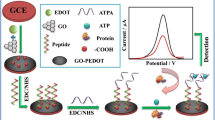
Abstract
An electrochemical sensor that can resist biofouling even when operated in complex biological medium is developed for the determination of dopamine. It is based on the use of the conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) that is doped with the water insoluble ionic liquid (IL), 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. A glassy carbon electrode modified with PEDOT/IL is shown to enable accurate determination of dopamine, as a model analyte in the presence of high concentrations of proteins, and resist biological fouling even in native serum. It exhibited a low limit of detection of 33 nM for the detection of dopamine, with a wide linear range from 0.2 to 328 μM (at 0.2 V vs. saturated calomel electrode). The PEDOT/IL modified glassy carbon electrode has a porous microstructure, high electrical conductivity and good stability. The sensor can be used to quantify dopamine in human urine samples with satisfying accuracy.

An antifouling electrochemical sensor capable of detecting target in complex biological samples was developed based on the use of a conducting polymer (PEDOT) that was doped with a water insoluble ionic liquid.





Similar content being viewed by others
References
Chang Y, Liao SC, Higuchi A, Ruaan RC, Chu CW, Chen WY (2008) A highly stable nonbiofouling surface with well-packed grafted zwitterionic polysulfobetaine for plasma protein repulsion. Langmuir 24:5453–5458
Cao B, Li LL, Wu HY, Tang Q, Sun BB, Dong H, Zhe J, Cheng G (2014) Zwitteration of dextran: a facile route to integrate antifouling, switchability and optical transparency into natural polymers. Chem Commun 50:3234–3237
Barfidokht A, Gooding JJ (2014) Approaches toward allowing electroanalytical devices to be used in biological fluids. Electroanalysis 26:1182–1196
Taufik S, Barfidokht A, Alam MT, Jiang C, Parker SG, Gooding JJ (2016) An antifouling electrode based on electrode–organic layer–nanoparticle constructs: electrodeposited organic layers versus self-assembled monolayers. J Electroanal Chem 779:229–235
Zhang ZG, Zheng JP, Zhang Y, Zhang W, Li LJ, Cao ZZ, Wang H, Li CH, Gao YF, Liu JR (2013) Anti-fouling in situ deposited antimony/nafion film electrode for electrochemical stripping analysis. Int J Electrochem Sci 8:4183–4193
Heli H, Sattarahmady N, Jabbari A, Moosavi-Movahedi AA, Hakimelahi GH, Tsai FY (2007) Adsorption of human serum albumin onto glassy carbon surface–applied to albumin-modified electrode: mode of protein–ligand interactions. J Electroanal Chem 610:67–74
Wang GX, Xu Q, Liu L, Su XL, Lin JH, Xu GX, Luo XL (2017) Mixed self-assembly of polyethylene glycol and aptamer on polydopamine surface for highly sensitive and low-fouling detection of adenosine triphosphate in complex media. ACS Appl Mater Interfaces 9:31153–31160
Das J, Huh CH, Kwon K, Park S, Jon S, Kim K, Yang H (2008) Comparison of the nonspecific binding of DNA-conjugated gold nanoparticles between polymeric and monomeric self-assembled monolayers. Langmuir 25:235–241
Sun K, Song LS, Xie YY, Liu DB, Wang D, Wang Z, Ma WS, Zhu JS, Jiang XY (2011) Using self-polymerized dopamine to modify the antifouling property of oligo (ethylene glycol) self-assembled monolayers and its application in cell patterning. Langmuir 27:5709–5712
Gui AL, Luais E, Peterson JR, Gooding JJ (2013) Zwitterionic phenyl layers: finally, stable, anti-biofouling coatings that do not passivate electrodes. ACS Appl Mater Interfaces 5:4827–4835
Ostuni E, Chapman RG, Holmlin RE, Takayama S, Whitesides GM (2001) A survey of structure− property relationships of surfaces that resist the adsorption of protein. Langmuir 17:5605–5620
Khor SM, Liu G, Fairman C, Iyengar SG, Gooding JJ (2011) The importance of interfacial design for the sensitivity of a label-free electrochemical immuno-biosensor for small organic molecules. Biosens Bioelectron 26:2038–2044
Wang GZ, Wang LG, Lin WF, Wang Z, Zhang J, Ji FQ, Ma GL, Yuan ZF, Chen SF (2015) Development of robust and recoverable ultralow-fouling coatings based on poly (carboxybetaine) ester analogue. ACS Appl Mater Interfaces 7:16938–16945
Luo XL, Weaver CL, Tan SS, Cui XT (2013) Pure graphene oxide doped conducting polymer nanocomposite for bio-interfacing. J Mater Chem B 1:1340–1348
Liu NZ, Hui N, Davis JJ, Luo XL (2018) Low fouling protein detection in complex biological media supported by a designed multifunctional peptide. ACS Sens 3:1210–1216
Nowinski AK, Sun F, White AD, Keefe AJ, Jiang S (2012) Sequence, structure, and function of peptide self-assembled monolayers. J Am Chem Soc 134:6000–6005
Bryan T, Luo X, Bueno PR, Davis JJ (2013) An optimised electrochemical biosensor for the label-free detection of C-reactive protein in blood. Biosens Bioelectron 39:94–98
Umek RM, Lin SW, Vielmetter J, Terbrueggen RH, Irvine B, Yu CJ, Kayyem J, Yowanto H, Blackburn GF, Farkas DH, Chen YP (2001) Electronic detection of nucleic acids: a versatile platform for molecular diagnostics. J Mol Diagn 3:74–84
Schilp S, Kueller A, Rosenhahn A, Grunze M, Pettitt ME, Callow ME, Callow JA (2007) Settlement and adhesion of algal cells to hexa (ethylene glycol)-containing self-assembled monolayers with systematically changed wetting properties. Biointerphases 2:143–150
Choi S, Murphy WL (2008) Multifunctional mixed SAMs that promote both cell adhesion and noncovalent DNA immobilization. Langmuir 24:6873–6880
Crispin X, Jakobsson FLE, Crispin A, Grim PCM, Andersson P, Volodin A, Haesendonck CV, Auweraer MVD, Salaneck WR, Berggren M (2006) The origin of the high conductivity of poly (3, 4-ethylenedioxythiophene)− poly (styrenesulfonate)(PEDOT− PSS) plastic electrodes. Chem Mater 18:4354–4360
Roncali J, Blanchard P, Frère P (2005) 3, 4-Ethylenedioxythiophene (EDOT) as a versatile building block for advanced functional π-conjugated systems. J Mater Chem 15:1589–1610
Selvaganesh SV, Mathiyarasu J, Phani KLN, Yegnaraman V (2007) Chemical synthesis of PEDOT–Au nanocomposite. Nanoscale Res Lett 2:546–549
Luo XL, Xu M, Freeman C, James TJ, Davis JJ (2013) Ultrasensitive label free electrical detection of insulin in neat blood serum. Anal Chem 85:4129–4134
Liu S, Ma YH, Zhang RQ, Luo XL (2016) Three-dimensional Nanoporous conducting polymer poly (3, 4-ethylenedioxythiophene)(PEDOT) decorated with copper nanoparticles: electrochemical preparation and enhanced nonenzymatic glucose sensing. Chem Electro Chem 3:1799–1804
Sheng G, Xu GY, Xu SH, Wang SY, Luo XL (2015) Cost-effective preparation and sensing application of conducting polymer PEDOT/ionic liquid nanocomposite with excellent electrochemical properties. RSC Adv 5:20741–20746
Lu L, Wu GH, Dong YJ, Wang JW, Bai GL (2016) Green and facile preparation of self-supporting nanoporous gold electrode and effect of ionic liquids on its electrocatalytic oxidation toward glucose. J Porous Mater 23:671–678
Xie ZC, Sun XF, Jiao JM, Xin X (2017) Ionic liquid-functionalized carbon quantum dots as fluorescent probes for sensitive and selective detection of iron ion and ascorbic acid. Colloids Surf A Physicochem Eng Asp 529:38–44
Liu XH, Bu CH, Nan ZH, Zheng LC, Qiu Y, Lu XQ (2013) Enzymes immobilized on amine-terminated ionic liquid-functionalized carbon nanotube for hydrogen peroxide determination. Talanta 105:63–68
Daggumati P, Matharu Z, Wang L, Seker E (2015) Biofouling-resilient nanoporous gold electrodes for DNA sensing. Anal Chem 87:8618–8622
Moulton SE, Barisci JN, Bath A, Stella R, Wallace GG (2003) Investigation of protein adsorption and electrochemical behavior at a gold electrode. J Colloid Interface Sci 261:312–319
Moulton SE, Barisci JN, Bath A, Stella R, Wallace GG (2004) Studies of double layer capacitance and electron transfer at a gold electrode exposed to protein solutions. Electrochim Acta 49:4223–4230
Jiang C, Tanzirul Alam M, Parker SG, Gooding JJ (2015) Zwitterionic phenyl Phosphorylcholine on indium tin oxide: a low-impedance protein-resistant platform for biosensing. Electroanalysis 27:884–889
Li J, Xia JF, Zhang FF, Wang ZH, Liu QY (2018) A novel electrochemical sensor based on copper-based metal-organic framework for the determination of dopamine. J Chin Chem Soc 65(6):743–749
He WY, Ding Y, Zhang WQ, Ji LF, Zhang X, Yang FC (2016) A highly sensitive sensor for simultaneous determination of ascorbic acid, dopamine and uric acid based on ultra-small Ni nanoparticles. J Electroanal Chem 775:205–211
Teymourian H, Salimi A, Khezrian S (2013) Fe3O4 magnetic nanoparticles/reduced graphene oxide nanosheets as a novel electrochemical and bioeletrochemical sensing platform. Biosens Bioelectron 49(15):1–8
Wang Y, Bi CY (2013) Simultaneous electrochemical determination of ascorbic acid, dopamine and uric acid using poly (tyrosine)/functionalized multi-walled carbon nanotubes composite film modified electrode. J Mol Liq 177:26–31
Wang X, Wang QX, Wang QH, Gao F, Gao F, Yang YZ, Guo HX (2014) Highly dispersible and stable copper terephthalate metal–organic framework–graphene oxide nanocomposite for an electrochemical sensing application. ACS Appl Mater Interfaces 6(14):11573–11580
Cai WH, Lai T, Du HJ, Ye JS (2014) Electrochemical determination of ascorbic acid, dopamine and uric acid based on an exfoliated graphite paper electrode: a high performance flexible sensor. Sensors Actuators B Chem 193(31):492–500
Ensafi AA, Taei M, Khayamian T, Arabzadeh A (2010) Highly selective determination of ascorbic acid, dopamine, and uric acid by differential pulse voltammetry using poly (sulfonazo III) modified glassy carbon electrode. Sensors Actuators B Chem 147(1):213–221
Lian QW, He ZF, He Q, Luo A, Yan KW, Zhang DX, Lu XQ, Zhou XB (2014) Simultaneous determination of ascorbic acid, dopamine and uric acid based on tryptophan functionalized graphene. Anal Chim Acta 823(1):32–39
Chen LX, Zheng JN, Wang AJ, Wu LJ, Chen JR, Feng JJ (2015) Facile synthesis of porous bimetallic alloyed PdAg nanoflowers supported on reduced graphene oxide for simultaneous detection of ascorbic acid, dopamine, and uric acid. Analyst 140(9):3183–3192
Zhang R, Jin GD, Chen D, Hu XY (2009) Simultaneous electrochemical determination of dopamine, ascorbic acid and uric acid using poly (acid chrome blue K) modified glassy carbon electrode. Sensors Actuators B Chem 138(1):174–181
Li YC, Jiang YY, Song YY, Li YH, Li SX (2018) Simultaneous determination of dopamine and uric acid in the presence of ascorbic acid using a gold electrode modified with carboxylated graphene and silver nanocube functionalized polydopamine nanospheres. Microchim Acta 185(8):382
Liu JC, Xie YZ, Wang K, Zeng QT, Liu R, Liu XY (2017) A nanocomposite consisting of carbon nanotubes and gold nanoparticles in an amphiphilic copolymer for voltammetric determination of dopamine, paracetamol and uric acid. Microchim Acta 184(6):1739–1745
Khan MZH, Liu XQ, Tang YF, Zhu JH, Hu WP, Liu XH (2018) A glassy carbon electrode modified with a composite consisting of gold nanoparticle, reduced graphene oxide and poly (L-arginine) for simultaneous voltammetric determination of dopamine, serotonin and L-tryptophan. Microchim Acta 185(9):439
Zhuang XM, Chen DD, Zhang S, Luan F, Chen LG (2018) Reduced graphene oxide functionalized with a CoS2/ionic liquid composite and decorated with gold nanoparticles for voltammetric sensing of dopamine. Microchim Acta 185(3):166
Raj M, Gupta P, Goyal RN, Shim YB (2017) Graphene/conducting polymer nano-composite loaded screen printed carbon sensor for simultaneous determination of dopamine and 5-hydroxytryptamine. Sensors Actuators B Chem 239:993–1002
Mei LP, Feng JJ, Wu L, Chen JR, Shen L, Xie Y, Wang AJ (2016) A glassy carbon electrode modified with porous Cu2O nanospheres on reduced graphene oxide support for simultaneous sensing of uric acid and dopamine with high selectivity over ascorbic acid. Microchim Acta 183:2039–2046
Cui M, Wang Y, Jiao MX, Jayachandran S, Wu YM, Fan XJ, Luo XL (2017) Mixed self-assembled aptamer and newly designed zwitterionic peptide as antifouling biosensing interface for electrochemical detection of alpha-fetoprotein. ACS Sens 2:490–494
Acknowledgements
This work is supported by the National Natural Science Foundation of China (21675093, 21422504), the Natural Science Foundation of Shandong Province of China (JQ201406), and the Taishan Scholar Program of Shandong Province of China (ts20110829).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 5.38 MB)
Rights and permissions
About this article
Cite this article
Song, Z., Sheng, G., Cui, Y. et al. Low fouling electrochemical sensing in complex biological media by using the ionic liquid-doped conducting polymer PEDOT: application to voltammetric determination of dopamine. Microchim Acta 186, 220 (2019). https://doi.org/10.1007/s00604-019-3340-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3340-x