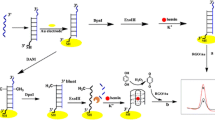
Abstract
A graphene oxide (GO)-based cost-effective, automatted strip test has developed for screening of inhibitors of endonuclease EcoRV. The method involves the use of GO and a DNA substrate for EcoRV that contains both an ssDNA region for binding of GO and a fluorescein amidite (FAM)-labelled dsDNA. All the components were inkjet printed on a piece of parchment paper. The ssDNA region binds to the surface of GO and anchors so that the fluorescence of FAM is quenched. The parchment paper strip is then incubated with a sample containing EcoRV which causes enzymatic hydrolysis, and dsDNA was separated from the GO. As a result, green fluorescence is generated at the reaction spot. Enzyme activity can be measured in the presence and absence of aurintricarboxy acid acting as an EcoRV inhibitor. This method excels by its need for 2–3 orders less reagents compared to the standard well plate assay. Thus, it is an efficient platform for GO-based screening of EcoRV enzyme inhibitors.

A graphene oxide (GO)-based endonuclease EcoRV inhibition FRET assay using inkjet printing was developed. Printing of GO along with assay reagents has a beneficial effect on the enzymatic reaction on paper. This method was successfully applied to evaluate EcoRV inhibitor activity.





Similar content being viewed by others
References
Morales-Narváez E, Merkoçi A (2012) Graphene oxide as an optical biosensing platform. Adv Mater 24(25):3298–3308
Chen P, Li N, Chen X, Ong WJ, Zhao X (2017) The rising star of 2D black phosphorus beyond graphene: synthesis, properties and electronic applications. 2D Mater 5(1):014002
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:11–19
Le Lay G, Salomon E, De Padova P, Layet JM, Angot T (2014) The rise of elemental two-dimensional materials beyond graphene. Aust J Chem 67(10):1370–1372
Labroo P, Cui Y (2013) Electrical, enzymatic graphene biosensing of 5-aminosalicylic acid. Analyst 138(5):1325–1328
Lee CY, Lei KF, Tsai SW, Tsang NM (2016) Development of graphene-based sensors on paper substrate for the measurement of ph value of analyte. BioChip J 10(3):182–188
Palanisamy S, Ku S, Chen SM (2013) Dopamine sensor based on a glassy carbon electrode modified with a reduced graphene oxide and palladium nanoparticles composite. Microchim Acta 180(11–12):1037–1042
Labroo P, Cui Y (2014) Graphene nano-ink biosensor arrays on a microfluidic paper for multiplexed detection of metabolites. Anal Chim Acta 813:90–96
Ruecha N, Rangkupan R, Rodthongkum N, Chailapakul O (2014) Novel paper-based cholesterol biosensor using graphene/polyvinylpyrrolidone/polyaniline nanocomposite. Biosens Bioelectron 52:13–19
Luo M, Chen X, Zhou G, Xiang X, Chen L, Ji X, He Z (2012) Chemiluminescence biosensors for DNA detection using graphene oxide and a horseradish peroxidase-mimicking DNAzyme. Chem Commun 48(8):1126–1128
Teymourian H, Salimi A, Khezrian S (2013) Fe3O4 magnetic nanoparticles/reduced graphene oxide nanosheets as a novel electrochemical and bioeletrochemical sensing platform. Biosens Bioelectron 49:1–8
Zhang Y, Chen X, Roozbahani GM, Guan X (2018) Graphene oxide-based biosensing platform for rapid and sensitive detection of HIV-1 protease. Anal Bioanal Chem 410(24):6177–6185
Bhunia SK, Jana NR (2011) Peptide-functionalized colloidal graphene via interdigited bilayer coating and fluorescence turn-on detection of enzyme. ACS Appl Mater Interfaces 3(9):3335–3341
Li J, Lu CH, Yao QH, Zhang XL, Liu JJ, Yang HH, Chen GN (2011) A graphene oxide platform for energy transfer-based detection of protease activity. Biosens Bioelectron 26(9):3894–3899
Liu Y, Luo M, Xiang X, Chen C, Ji X, Chen L, He Z (2014) A graphene oxide and exonuclease-aided amplification immuno-sensor for antigen detection. Chem Commun 50(20):2679–2681
Lu Z, Chen X, Wang Y, Zheng X, Li CM (2015) Aptamer based fluorescence recovery assay for aflatoxin B1 using a quencher system composed of quantum dots and graphene oxide. Microchim Acta 182(3–4):571–578
Chang H, Tang L, Wang Y, Jiang J, Li J (2010) Graphene fluorescence resonance energy transfer aptasensor for the thrombin detection. Anal Chem 82(6):2341–2346
Dong H, Zhang J, Ju H, Lu H, Wang S, Jin S, Hao K, Du H, Zhang X (2012) Highly sensitive multiple microRNA detection based on fluorescence quenching of graphene oxide and isothermal strand-displacement polymerase reaction. Anal Chem 84(10):4587–4593
Jang H, Ryoo SR, Kim YK, Yoon S, Kim H, Han SW, Choi BS, Kin DE, Min DH (2013) Discovery of hepatitis C virus NS3 helicase inhibitors by a multiplexed, high-throughput helicase activity assay based on graphene oxide. Angew Chem Int Ed Engl 125(8):2396–2400
Yin Z, He Q, Huang X, Zhang H et al (2012) Real-time DNA detection using Pt nanoparticle-decorated reduced graphene oxide field-effect transistors. Nanoscale 4(1):293–297
Giardi R, Porro S, Chiolerio A, Celasco E, Sangermano M (2013) Inkjet printed acrylic formulations based on UV-reduced graphene oxide nanocomposites. J Mater Sci 48(3):1249–1255
Huang L, Huang Y, Liang J, Wan X, Chen Y (2011) Graphene-based conducting inks for direct inkjet printing of flexible conductive patterns and their applications in electric circuits and chemical sensors. Nano Res 4(7):675–684
Mei Q, Zhang Z (2012) Photoluminescent graphene oxide ink to print sensors onto microporous membranes for versatile visualization bioassays. Angew Chem Int Ed Engl 51(23):5602–5606
Wisitsoraat A, Mensing JP, Karuwan C, Tuantranont A et al (2017) Printed organo-functionalized graphene for biosensing applications. Biosens Bioelectron 87:7–17
Ping J, Wang Y, Fan K, Wu J, Ying Y (2011) Direct electrochemical reduction of graphene oxide on ionic liquid doped screen-printed electrode and its electrochemical biosensing application. Biosens Bioelectron 28(1):204–209
Wisitsoraat A, Pakapongpan S, Sriprachuabwong C, Phokharatkul D, Sritongkham P, Lomas T, Tuantranont A (2013) Graphene–PEDOT: PSS on screen printed carbon electrode for enzymatic biosensing. J Electroanal Chem 704:208–213
Wu Y, Xue P, Hui KM, Kang Y (2014) A paper-based microfluidic electrochemical immunodevice integrated with amplification-by-polymerization for the ultrasensitive multiplexed detection of cancer biomarkers. Biosens Bioelectron 52:180–187
Filipponi L, Livingston P, Kašpar O, Tokárová V, Nicolau DV (2016) Protein patterning by microcontact printing using pyramidal PDMS stamps. Biomed Microdevices 18(1):9–16
Castagna R, Bertucci A, Prasetyanto EA, Monticelli M, Conca DV, Massetti M, Bertacco R (2016) Reactive microcontact printing of DNA probes on (DMA-NAS-MAPS) copolymer-coated substrates for efficient hybridization platforms. Langmuir 32(13):3308–3313
Kobayashi R, Biyani M, Ueno S, Kumal SR, Kuramochi H, Ichiki T (2015) Temperature-controlled microintaglio printing for high-resolution micropatterning of RNA molecules. Biosens Bioelectron 67:115–120
Hutama TJ, Oleschuk RD (2017) Magnetically manipulated droplet splitting on a 3D-printed device to carry out a complexometric assay. Lab Chip 17(15):2640–2649
Lamas-Ardisana PJ, Martínez-Paredes G, Añorga L, Grande HJ (2018) Glucose biosensor based on disposable electrochemical paper-based transducers fully fabricated by screen-printing. Biosens Bioelectron 109:8–12
Jeon S, Lee JP, Kim JM (2015) In situ synthesis of stimulus-responsive luminescent organic materials using a reactive inkjet printing approach. Journal of Materials Chemistry C 3(12):2732–2736
Choi W, Harshey RM (2010) DNA repair by the cryptic endonuclease activity of Mu transposase. Proc Natl Acad Sci U S A 107(22):10014–10019
Huang KJ, Ku CC, Lehman IR (2006) Endonuclease G: a role for the enzyme in recombination and cellular proliferation. Proc Natl Acad Sci U S A 103(24):8995–9000
Lee J, Kim YK, Min DH (2011) A new assay for endonuclease/methyltransferase activities based on graphene oxide. Anal Chem 83(23):8906–8912
Ma L, Zhu Z, Li T, Wang Z (2014) Assaying multiple restriction endonucleases functionalities and inhibitions on DNA microarray with multifunctional gold nanoparticle probes. Biosens Bioelectron 52:118–123
Luo JY, Cote LJ, Tung VC, Tan ATL, Goins PE, Wu JS, Jiaxing H (2010) Graphene oxide nanocolloids. J Am Chem Soc 132(50):17667–17669
Lee J, Samson AAS, Song JM (2017) Inkjet-printing enzyme inhibitory assay based on determination of ejection volume. Anal Chem 89(3):2009–2016
He Y, Cheng F, Pang DW, Tang HW (2017) Colorimetric and visual determination of DNase I activity using gold nanoparticles as an indicator. Microchim Acta 184:101–106
Ji L, Qian Y, Wu P, Zhang H, Cai C (2015) Fluorescence quenching of graphene oxide combined with the site-specific cleavage of restriction endonuclease for deoxyribonucleic acid demethylase activity assay. Anal Chim Acta 869:74–80
Huang LJ, Tian X, Yi JT, Yu RQ, Chu X (2015) A turn-on upconversion fluorescence resonance energy transfer biosensor for ultrasensitive endonuclease detection. Anal Methods 7:7474–7479
Mao S, Lu G, Yu K, Bo Z, Chen J (2010) Specific protein detection using thermally reduced graphene oxide sheet decorated with gold nanoparticle-antibody conjugates. Adv Mater 22(32):3521–3526
Wang H, Zhang Q, Chu X, Chen T, Ge J, Yu R (2011) Graphene oxide–peptide conjugate as an intracellular protease sensor for caspase-3 activation imaging in live cells. Angew Chem Int Ed Engl 123(31):7203–7207
Acknowledgements
This work was supported by National Research Foundation of Korea (NRF) grant funded by the Ministry of Education, Science and Technology (MEST) (2016R1A4A1010796 and 2018M3A7B4071235). We are grateful to the Research Institute of Pharmaceutical Sciences at Seoul National University for providing experimental equipment and Brain Korea 21 plus (BK 21 plus). The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 24.0 kb)
Rights and permissions
About this article
Cite this article
Lee, J., Samson, A.A.S., Yim, Y. et al. A FRET assay for the quantitation of inhibitors of exonuclease EcoRV by using parchment paper inkjet-printed with graphene oxide and FAM-labelled DNA. Microchim Acta 186, 211 (2019). https://doi.org/10.1007/s00604-019-3317-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3317-9