Abstract
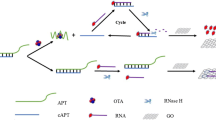
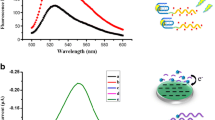
A fluorometric assay is described for ochratoxin A (OTA) using an aptamer. The method is based on exonuclease-assisted recycling amplification. The OTA-binding aptamer partially hybridizes with complementary DNA (cDNA) that is released when the aptamer recognizes OTA. Then, cDNA hybridizes with a specifically designed hairpin DNA. Next, short ssDNA and cDNA are, respectively, released by exonuclease III catalyzed hydrolysis of the dsDNA. The cDNA induces the next ring opening and digestion. The short ssDNA captures the sDNA that is labeled with fluorescent FAM and is absorbed on graphene oxide (GO). The green fluorescence of the sDNA/GO system is quenched but is recovered if the sDNA is released from GO. This assay is high sensitive, works in the 5 nM to 200 nM OTA concentration range and has a 0.96 nM lower detection limit. It was applied to the quantitation of OTA in spiked wine and coffee samples.

Schematic of a fluorometric assay based on exonuclease-assisted recycling amplification for quantitative monitoring of OTA without the need of sample separation and multiple washing steps.




Similar content being viewed by others
References
Petzinger E, Ziegler K (2000) Ochratoxin A from a toxicological perspective. J Vet Pharmacol Ther 23:91–98. https://doi.org/10.1046/j.1365-2885.2000.00244.x
Pfohl AL, Manderville RA (2007) Ochratoxin A: an overview on toxicity and carcinogenicity in animals and humans. Mol Nutr Food Res 51:61–99. https://doi.org/10.1002/mnfr.200600137
Yin J, Liu Y, Wang S (2018) Engineering a universal and label-free evaluation method for mycotoxins detection based on strand displacement amplification and G-quadruplex signal amplification. Sensor Actuat B-Chem 256:573–579. https://doi.org/10.1016/j.snb.2017.10.083
Mcmahon G, Hanson L, Lee JJ (1986) Identification of an activated c-Ki-ras oncogene in rat liver tumors induced by aflatoxin B1. Proc Natl Acad Sci U S A 83:9418–9422. https://doi.org/10.1073/pnas.83.24.9418
Brien EO, Dietrich DR (2005) Ochratoxin A: the continuing enigma. Crit Rev Toxicol 35:33–60. https://doi.org/10.1080/10408440590905948
Horvath A, Upham LB, Ganev V (2002) Determination of the epigenetic effects of ochratoxin in a human kidney and a rat liver epithelial cell line. Toxicon 40:273–282. https://doi.org/10.1016/S0041-0101(01)00219-7
European Commission (2006) Commission regulation (EC) no 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union L364:5e24
Soleas GJ, Yan J, Goldberg DM (2001) Assay of ochratoxin A in wine and beer by high-pressure liquid chromatography photodiode array and gas chromatography mass selective detection. J Agric Food Chem 49:2733–2740. https://doi.org/10.1021/jf0100651
Liang Y, Huang X, Chen X (2018) Plasmonic ELISA for naked-eye detection of ochratoxin A based on the tyramine-H2O2 amplification system. Sensor Actuat B-Chem 259:162–169. https://doi.org/10.1016/j.snb.2017.12.004
Qin X, Wang Q, Geng L (2019) A “signal-on” photoelectrochemical aptasensor based on graphene quantum dots-sensitized TiO2 nanotube arrays for sensitive detection of chloramphenicol. Talanta 197:28–35. https://doi.org/10.1016/j.talanta.2018.12.103
Jiang Y, Sun W-D, Pu H (2019) Ultrasensitive analysis of kanamycin residue in milk by SERS-based aptasensor. Talanta 197:151–158. https://doi.org/10.1016/j.talanta.2019.01.015
Zhou L, Ji F, Zhang T (2019) An fluorescent aptasensor for sensitive detection of tumor marker based on the FRET of a sandwich structured QDs-AFP-AuNPs. Talanta 197:444–450. https://doi.org/10.1016/j.talanta.2019.01.012
Chen X, Huang Y, Duan N (2014) Selection and characterization of single stranded DNA aptamers recognizing fumonisin B-1. Microchim Acta 81:11–12. https://doi.org/10.1007/s00604-014-1260-3
Yuan F, Chen H, Xu J (2014) Aptamer-based luminescence energy transfer from near-infrared-to-near-infrared upconverting nanoparticles to gold nanorods and its application for the detection of thrombin. Chem Eur J 20:2888–2894. https://doi.org/10.1002/chem.201304556
Ma C, Wu K, Zhao H, Liu H, Wang K, Xia K (2018) Fluorometric aptamer-based determination of ochratoxin A based on the use of graphene oxide and RNase H-aided amplification. Microchim Acta 185(7):347. https://doi.org/10.1007/s00604-018-2885-4
Liu R, Wu H, Lv L, Kang X, Cui C, Feng J, Guo Z (2018) Fluorometric aptamer based assay for ochratoxin A based on the use of exonuclease III. Microchim Acta 185(5):254. https://doi.org/10.1007/s00604-018-2786-6
Wu H, Liu R, Kang X, Liang C, Lv L, Guo Z (2018) Fluorometric aptamer assay for ochratoxin A based on the use of single walled carbon nanohorns and exonuclease III-aided amplification. Microchim Acta 185(1):27–27. https://doi.org/10.1007/s00604-017-2592-6
Yu X, Lin Y, Wang X, Xu L, Wang Z, Fu F (2018) Exonuclease-assisted multicolor aptasensor for visual detection of ochratoxin A based on G-quadruplex-hemin DNAzyme-mediated etching of gold nanorod. Microchim Acta 185(5):259. https://doi.org/10.1007/s00604-018-2811-9
Yang C, Wang Y, Martyc JL, Yang X-R (2011) Aptamer-based colorimetric biosensing of Ochratoxin A using unmodified gold nanoparticles indicator. Biosens Bioelectron 26:2724–2727. https://doi.org/10.1016/j.bios.2010.09.032
Dai S-L, Wu S-J, Duan N, Wang Z-P (2016) A near-infrared magnetic aptasensor for ochratoxin A based on near-infrared upconversion nanoparticles and magnetic nanoparticles. Talanta 158:246–253. https://doi.org/10.1016/j.talanta.2016.05.063
Sun A-L, Zhang Y-F, Sun G-P, Wang X-N, Tang D-P (2017) Homogeneous electrochemical detection of ochratoxin A in foodstuff using aptamer-graphene oxide nanosheets and DNase I-based target recycling reaction. Biosens Bioelectron 89:659–665. https://doi.org/10.1016/j.bios.2015.12.032
Zhang X, Wu D, Liu Z (2014) An ultrasensitive label-free electrochemical biosensor for microRNA-21 detection based on a 2′-O-methyl modified DNAzyme and duplex-specific nuclease assisted target recycling. Chem Commun 50:12375–12377. https://doi.org/10.1039/c4cc05541a
Liu Q, Liu C, Zhu G (2019) Electrochemiluminescent determination of the activity of uracil-DNA glycosylase: combining nicking enzyme assisted signal amplification and catalyzed hairpin assembly. Microchim Acta 186:4–10. https://doi.org/10.1007/s00604-019-3280-5
Wang H-F, Ma R-N, Sun F (2018) A versatile label-free electrochemical biosensor for circulating tumor DNA based on dual enzyme assisted multiple amplification strategy. Biosens Bioelectron 122:224–230. https://doi.org/10.1016/j.bios.2018.09.028
Yan M-M, Bai W-H, Zhu C, Huang Y-F, Yan J, Chen A-L (2016) Design of nuclease-based target recycling signal amplification in aptasensors. Biosens Bioelectron 77:613–623. https://doi.org/10.1016/j.bios.2015.10.015
Jo E-J, Mun H, Kim S-J, Shim W-B, Kim M-G (2016) Detection of ochratoxin A (OTA) in coffee using chemiluminescence resonance energy transfer (CRET) aptasensor. Food Chem 194:1102–1107. https://doi.org/10.1016/j.foodchem.2015.07.152
Cruz JA, Penner G (2008) Fluorescence polarization based displacement assay for the determination of small molecules with aptamers. Anal Chem 80:8853–8855. https://doi.org/10.1021/ac8017058
Klug SJ, Famulok M (1994) All you wanted to know about SELEX. Mol Biol Rep 20:97–107. https://doi.org/10.1007/BF00996358
Cruz JA, Penner G (2008) Determination of ochratoxin A with a DNA aptamer. J Agr Food Chem 56:10456–10461. https://doi.org/10.1021/jf801957h
Holm AIS, Kohler B, Hoffmann SV (2010) Synchrotron radiation circular dichroism of various G-guadruplex structures. Biopolymers 93:429–433. https://doi.org/10.1002/bip.21354
Dong H, Chen H, Jiang J (2018) Highly sensitive electrochemical detection of tumor exosomes based on aptamer recognition-induced multi-DNA release and cyclic enzymatic amplification. Anal Chem 90:4507–4513. https://doi.org/10.1021/acs.analchem.7b04863
Sheng L, Ren J, Miao Y, Wang J, Wang E (2011) PVP-coated graphene oxide for selective determination of ochratoxin A via quenching fluorescence of free aptamer. Biosens Bioelectron 26(8):3494–3499. https://doi.org/10.1016/j.bios.2011.01.032
Liu Y, Yan H, Shangguan J, Yang X, Wang M, Liu W (2018) A fluorometric aptamer-based assay for ochratoxin A using magnetic separation and a cationic conjugated fluorescent polymer. Microchim Acta 185(9):427. https://doi.org/10.1007/s00604-018-2962-8
Lv L, Cui C, Liang C, Quan W, Wang S, Guo Z (2016) Aptamer-based single-walled carbon nanohorn sensors for ochratoxin A detection. Food Control 60:296–301. https://doi.org/10.1016/j.foodcont.2015.08.002
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (No. GK201802012) and Shaanxi Science Technology Plan Projects (No. 2017NY-121).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 15174 kb)
Rights and permissions
About this article
Cite this article
Liu, M., Li, X., Li, B. et al. A fluorometric aptamer-based assay for ochratoxin A by using exonuclease III-assisted recycling amplification. Microchim Acta 187, 46 (2020). https://doi.org/10.1007/s00604-019-3992-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3992-6