Abstract
Endotoxins are complex lipopolysaccharides (LPS) and key components of the outer cell membrane of Gram-negative bacteria. The authors report on a fluorescent aptamer-based probe for the determination of LPS of Gram-negative bacteria. An aptamer against LPS was fluorescently labeled with CdSe/ZnS quantum dots. Its emission is quenched on addition of graphene oxide (GO). On addition of LPS, the aptamer binds LPS and GO is released. This results in the recovery of fluorescence, typically measured at excitation/emission wavelengths of 495/543 nm. The probe responds to LPS in the 10–500 ng·mL−1 concentration range, and the detection limit is 8.7 ng·mL−1. It can be used for selective detection of LPS from different Gram-negative bacteria, in the presence of biological interferents.

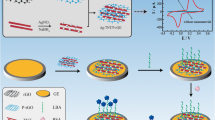
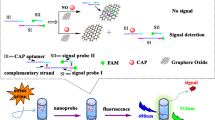
Schematic presentation of a green fluorescent probe comprised of an aptamer labelled with CdSe/ZnS quantum dots and of graphene oxide. Lipopolysaccharides bind to the aptamer and release graphene oxide to result in fluorescence recovery, which is measured at an emission wavelength 543 nm.





Similar content being viewed by others
References
Erridge C, Bennett-Guerrero E, Poxton IR (2002) Structure and function of lipopolysaccharides. Microbes Infect 4(8):837–851. https://doi.org/10.1016/s1286-4579(02)01604-0
Ulevitch RJ, Tobias PS (1995) Recptor-dependent michanisms of cell stimulation by bacterial-endotoxin. Annu Rev Immunol 13:437–457. https://doi.org/10.1146/annurev.iy.13.040195.002253
Cooper JF, Levin J, Wagner HN (1971) Quantitative comparison of in-vitro and in-vivo methods for detection of endotoxin. J Lab Clin Med 78(1):138–148
Bolden JS, Warburton RE, Phelan R, Murphy M, Smith KR, De Felippis MR, Chen D (2016) Endotoxin recovery using limulus amebocyte lysate (LAL) assay. Biologicals 44(5):434–440. https://doi.org/10.1016/j.biologicals.2016.04.009
Liu T, Zhang W, Zhou L, Guo Z, Tang Y, Miao P (2017) A quartz crystal microbalance sensor for endotoxin assay by monitoring limulus amebocyte lysate protease reaction. Anal Chim Acta 961:106–111. https://doi.org/10.1016/j.aca.2017.01.014
Das AP, Kumar PS, Swain S (2014) Recent advances in biosensor based endotoxin detection. Biosens Bioelectron 51:62–75. https://doi.org/10.1016/j.bios.2013.07.020
Alahi MEE, Mukhopadhyay SC (2017) Detection methodologies for pathogen and toxins: a review. Sensors 17(8). https://doi.org/10.3390/s17081885
Cheng C, Wu J, Chen J (2018) A highly sensitive aptasensor for on-site detection of lipopolysaccharides in food. Electrophoresis. https://doi.org/10.1002/elps.201800289
Ding S-J, Chang B-W, Wu C-C, Chen C-J, Chang H-C (2007) A new method for detection of endotoxin on polymyxin B-immobilized gold electrodes. Electrochem Commun 9(5):1206–1211. https://doi.org/10.1016/j.elecom.2006.12.029
Posha B, Nambiar SR, Sandhyarani N (2018) Gold atomic cluster mediated electrochemical aptasensor for the detection of lipopolysaccharide. Biosens Bioelectron 101:199–205. https://doi.org/10.1016/j.bios.2017.10.030
Ying G, Wang M, Yi Y, Chen J, Mei J, Zhang Y, Chen S (2018) Construction and application of an electrochemical biosensor based on an endotoxin aptamer. Biotechnol Appl Biochem 65(3):323–327. https://doi.org/10.1002/bab.1610
Liu T, Gao L, Zhao J, Cao Y, Tang Y, Miao P (2018) A polymyxin B-silver nanoparticle colloidal system and the application of lipopolysaccharide analysis. Analyst 143(5):1053–1058. https://doi.org/10.1039/c7an01788j
Zandieh M, Hosseini SN, Vossoughi M, Khatami M, Abbasian S, Moshaii A (2018) Label-free and simple detection of endotoxins using a sensitive LSPR biosensor based on silver nanocolumns. Anal Biochem 548:96–101. https://doi.org/10.1016/j.ab.2018.02.023
Wu J, Zawistowski A, Ehrmann M, Yi T, Schmuck C (2011) Peptide functionalized Polydiacetylene liposomes act as a fluorescent turn-on sensor for bacterial lipopolysaccharide. J Am Chem Soc 133(25):9720–9723. https://doi.org/10.1021/ja204013u
Jiang G, Wang J, Yang Y, Zhang G, Liu Y, Lin H, Zhang G, Li Y, Fan X (2016) Fluorescent turn-on sensing of bacterial lipopolysaccharide in artificial urine sample with sensitivity down to nanomolar by tetraphenylethylene based aggregation induced emission molecule. Biosens Bioelectron 85:62–67. https://doi.org/10.1016/j.bios.2016.04.071
Voss S, Fischer R, Jung G, Wiesmueller K-H, Brock R (2007) A fluorescence-based synthetic LPS sensor. J Am Chem Soc 129(3):554–561. https://doi.org/10.1021/ja065016p
Xie L, Ling X, Fang Y, Zhang J, Liu Z (2009) Graphene as a substrate to suppress fluorescence in resonance Raman spectroscopy. J Am Chem Soc 131(29):9890–9891. https://doi.org/10.1021/ja9037593
Lim SK, Chen P, Lee FL, Moochha S, Liedberg B (2015) Peptide-assembled graphene oxide as a fluorescent turn-on sensor for lipopolysaccharide (endotoxin) detection. Anal Chem 87(18):9408–9412. https://doi.org/10.1021/acs.analchem.5b02270
Zhang Z, Yang J, Pang W, Yan G (2017) An aptamer-based fluorescence probe for facile detection of lipopolysaccharide in drinks. RSC Adv 7(86):54920–54926. https://doi.org/10.1039/c7ra10710b
Mattoussi H, Mauro JM, Goldman ER, Anderson GP, Sundar VC, Mikulec FV, Bawendi MG (2000) Self-assembly of CdSe−ZnS quantum dot bioconjugates using an engineered recombinant protein. J Am Chem Soc 122(49):12142–12150. https://doi.org/10.1021/ja002535y
Zhen SJ, Zhuang HL, Wang J, Huang CZ (2013) Dual-aptamer-based sensitive and selective detection of prion protein through the fluorescence resonance energy transfer between quantum dots and graphene oxide. Anal Methods 5(24):6904–6907. https://doi.org/10.1039/c3ay41335g
Lu W, Qin X, Luo Y, Chang G, Sun X (2011) CdS quantum dots as a fluorescent sensing platform for nucleic acid detection. Microchim Acta 175(3–4):355–359. https://doi.org/10.1007/s00604-011-0657-5
Duan YF, Ning Y, Song Y, Deng L (2014) Fluorescent aptasensor for the determination of Salmonella typhimurium based on a graphene oxide platform. Microchim Acta 181(5–6):647–653. https://doi.org/10.1007/s00604-014-1170-4
Lu Z, Chen X, Wang Y, Zheng X, Li CM (2015) Aptamer based fluorescence recovery assay for aflatoxin B1 using a quencher system composed of quantum dots and graphene oxide. Microchim Acta 182(3–4):571–578. https://doi.org/10.1007/s00604-014-1360-0
Kim S-E, Su W, Cho M, Lee Y, Choe W-S (2012) Harnessing aptamers for electrochemical detection of endotoxin. Anal Biochem 424(1):12–20. https://doi.org/10.1016/j.ab.2012.02.016
Wang R, Xu Y, Sors T, Irudayaraj J, Ren W, Wang R (2018) Impedimetric detection of bacteria by using a microfluidic chip and silver nanoparticle based signal enhancement. Microchim Acta 185(3):184. https://doi.org/10.1007/s00604-017-2645-x
Cui F, Xu Y, Wang R, Liu H, Chen L, Zhang Q, Mu X (2017) Label-free impedimetric glycan biosensor for quantitative evaluation interactions between pathogenic bacteria and mannose. Biosens Bioelectron 103:94–98. https://doi.org/10.1016/j.bios.2017.11.068
Wang R, Ni Y, Xu Y, Jiang Y, Dong C, Chuan N (2015) Immuno-capture and in situ detection of Salmonella typhimurium on a novel microfluidic chip. Anal Chim Acta 853:710–717. https://doi.org/10.1016/j.aca.2014.10.042
Wang R, Xu Y, Zhang T, Jiang Y (2015) Rapid and sensitive detection of Salmonella typhimurium using aptamer-conjugated carbon dots as fluorescence probe. Anal Methods 7(5):1701–1706. https://doi.org/10.1039/c4ay02880e
Bai L, Chai Y, Pu X, Yuan R (2014) A signal-on electrochemical aptasensor for ultrasensitive detection of endotoxin using three-way DNA junction-aided enzymatic recycling and graphene nanohybrid for amplification. Nanoscale 6(5):2902–2908. https://doi.org/10.1039/c3nr05930h
Ye H, Duan N, Wu S, Tan G, Gu H, Li J, Wang H, Wang Z (2017) Orientation selection of broad-spectrum aptamers against lipopolysaccharides based on capture-SELEX by using magnetic nanoparticles. Microchim Acta 184(11):4235–4242. https://doi.org/10.1007/s00604-017-2453-3
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No.21375156), National High Technology Research and Development Program of China (Ministry of Science and Technology 863 Plan)(2015AA021104), Frontier Research Key Projects of Chongqing Science and Technology Committee (cstc2015jcyjBX0010), and Fundamental Research Funds for the Central Universities (No. 10611CDJXZ238826).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• A fluorescence probe was constructed in combination of QDs labeled aptamers with GO.
• The GO-aptamer-QD probe can recognize and bind with LPS, releasing GO and recovering fluorescence.
• The constructed probe can detect LPS in injections sensitively with low LOD 8.7 ng mL-1.
Electronic supplementary material
ESM 1
(DOCX 2446 kb)
Rights and permissions
About this article
Cite this article
Wen, Lx., Lv, Jj., Chen, L. et al. A fluorescent probe composed of quantum dot labeled aptamer and graphene oxide for the determination of the lipopolysaccharide endotoxin. Microchim Acta 186, 122 (2019). https://doi.org/10.1007/s00604-018-3218-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3218-3