Abstract
An electrochemical and colorimetric dual-readout method is described for the determination of thrombin. A platinum nanoparticle (Pt NP) modified metal organic framework (MOF) acts as a peroxidase (POx) mimic that causes the formation of a blue product from 3,3′,5,5′-tetramethylbenzidine (TMB) and hydrogen peroxide, with an absorption maximum at 650 nm. In addition, gold nanoparticles enrich initiators that trigger the hybridization chain reaction for dual signal amplification to generate an electrochemical current typically measured at 0.31 V (from −0.5 to −0.1 V) and allow quantitation of thrombin with high sensitivity and over a wide detection range. The colorimetric and electrochemical (dual) thrombin assay produces two kinds of signals which warrants accuracy, diversity, and an option for visual inspection. The dual-channel sensor allows for the quantitative determination of thrombin with a low detection limit (0.33 fM) for the electrochemical method and 0.17 pM for the colorimetric method) and over a wide detection range (1 fM to 10 nM for electrochemical method and 0.5 pM to 1 nM for colorimetric method). The electrochemical detection limit is lower than that of colorimetry, and the linear range is wider, which is more suitable for further quantitative analysis of the target.

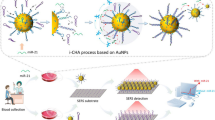
Schematic representation of a colorimetric and electrochemical (dual) thrombin assay based on the use of a platinum nanoparticle modified metal-organic framework for color development and hybridization chain reaction for electrochemical signal. C-TBA: complementary sequences of thrombin aptamer, TBA: thrombin aptamer, I-Au NPs: initiators enriched by gold nanoparticles, S-AuE: sensing gold electrode, RS-AuE: reacted sensing gold electrode, TB: thrombin, MB: Methylene Blue, HCR: hybridization chain reaction.







Similar content being viewed by others
References
Chen ZB, Tan Y, Zhang CM, Yin L, Ma H, Ye NS, Qiang H, Lin YQ (2014) A colorimetric aptamer biosensor based on cationic polymer and gold nanoparticles for the ultrasensitive detection of thrombin. Biosens Bioelectron 56:46-50. https://doi.org/10.1016/j.bios.2014.01.012
Bai LJ, Chai YQ, Pu XY, Yuan R (2014) A signal-on electrochemical aptasensor for ultrasensitive detection of endotoxin using three-way DNA junction-aided enzymatic recycling and graphene nanohybrid for amplification. Nanoscale 6(5):2902-2908. https://doi.org/10.1039/c3nr05930h
Qiu ZL, Shu J, Tang DP (2018) Near-infrared-to-ultraviolet light-mediated Photoelectrochemical Aptasensing platform for Cancer biomarker based on Core Shell NaYF4:Yb,tm@TiO2 Upconversion microrods. Anal Chem 90(1):1021-1028. https://doi.org/10.1021/acs.analchem.7b04479
Ouyang H, Lu Q, Wang WW, Song Y, Tu XM, Zhu CZ, Smith JN, Du D, Fu ZF, Lin YH (2018) Dual-readout Immunochromatographic assay by utilizing MnO2 Nanoflowers as the unique colorimetric/chemiluminescent probe. Anal Chem 90(8):5147-5152. https://doi.org/10.1021/acs.analchem.7b05247
Wu P, Miao LN, Wang HF, Shao XG, Yan XP (2011) A multidimensional sensing device for the discrimination of proteins based on manganese-doped ZnS quantum dots. Angew Chem Int Edit 50(35):8118-8121. https://doi.org/10.1002/anie.201101882
Chuong TT, Pallaoro A, Chaves CA, Li Z, Lee J, Eisenstein M, Stucky GD, Moskovits M, Soh HT (2017) Dual-reporter SERS-based biomolecular assay with reduced false-positive signals. P Natl Acad Sci USA 114(34):9056-9061. https://doi.org/10.1073/pnas.1700317114
Tang DP, Tang J, Li QF, Su BL, Chen GN (2011) Ultrasensitive aptamer-based multiplexed electrochemical detection by coupling distinguishable signal tags with catalytic recycling of DNase I. Anal Chem 83(19):7255-7259. https://doi.org/10.1021/ac201891w
Liu DB, Wang ZT, Jin A, Huang XL, Sun XL, Wang F, Yan Q, Ge SX, Xia NS, Niu G, Liu G, Walker ARH, Chen XY (2013) Acetylcholinesterase-catalyzed hydrolysis allows ultrasensitive detection of pathogens with the naked eye. Angew Chem Int Edit 52(52):14065-14069. https://doi.org/10.1002/anie.201307952
Wang ZZ, Chen ZW, Gao N, Ren JS, Qu XG (2015) Transmutation of personal glucose meters into portable and highly sensitive microbial pathogen detection platform. Small 11(37):4970-4975. https://doi.org/10.1002/smll.201500944
Liu DB, Chen WW, Wei JH, Li XB, Wang Z, Jiang XY (2012) A highly sensitive, dual-readout assay based on gold nanoparticles for organophosphorus and carbamate pesticides. Anal Chem 84(9):4185-4191. https://doi.org/10.1021/ac300545p
Deng JJ, Ma WJ, Yu P, Mao LQ (2015) Colorimetric and fluorescent dual mode sensing of alcoholic strength in Spirit samples with stimuli-responsive infinite coordination polymers. Anal Chem 87(13):6958-6965. https://doi.org/10.1021/acs.analchem.5b01617
Luo ZB, Zhang LJ, Zeng RJ, Su LS, Tang DP (2018) Near-infrared light-excited Core-Core-Shell UCNP@au@CdS Upconversion Nanospheres for ultrasensitive Photoelectrochemical enzyme immunoassay. Anal Chem 90(15):9568-9575. https://doi.org/10.1021/acs.analchem.8b02421
Tang DP, Lin YX, Zhou Q (2018) Carbon dots prepared from Litchi chinensis and modified with manganese dioxide nanosheets for use in a competitive fluorometric immunoassay for aflatoxin B-1. Microchim Acta 185(10):476. https://doi.org/10.1007/s00604-018-3012-2
Hou YH, Wang JJ, Jiang YZ, Lv C, Xia L, Hong SL, Lin M, Lin Y, Zhang ZL, Pang DW (2018) A colorimetric and electrochemical immunosensor for point-of-care detection of enterovirus 71. Biosens Bioelectron 99:186-192. https://doi.org/10.1016/j.bios.2017.07.035
Duan WN, Wang XZ, Wang HX, Li F (2018) Fluorescent and colorimetric dual-mode aptasensor for thrombin detection based on target-induced conjunction of split aptamer fragments. Talanta 180:76-80. https://doi.org/10.1016/j.talanta.2017.12.033
Wang YL, Sun YJ, Dai HC, Ni PJ, Jiang S, Lu WD, Li Z, Li Z (2016) A colorimetric biosensor using Fe3O4 nanoparticles for highly sensitive and selective detection of tetracyclines. Sensor Actuat B-Chem 236:621-626. https://doi.org/10.1016/j.snb.2016.06.029
Li YH, Liu XY, Zhang RY (2017) Sensitive and selective colorimetric detection of glutathione in human plasma with 2,2 '-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) and ag+ ion. Spectrochim Acta A 173:880-885. https://doi.org/10.1016/j.saa.2016.10.048
Ren RR, Cai GN, Yu ZZ, Zeng YY, Tang DP (2018) Metal-Polydopamine framework: an innovative signal-generation tag for colorimetric immunoassay. Anal Chem 90(18):11099-11105. https://doi.org/10.1021/acs.analchem.8b03538
Deng HH, Lin XL, Liu YH, Li KL, Zhuang QQ, Peng HP, Liu AL, Xia XH, Chen W (2017) Chitosan-stabilized platinum nanoparticles as effective oxidase mimics for colorimetric detection of acid phosphatase. Nanoscale 9(29):10292-10300. https://doi.org/10.1039/c7nr03399k
Peng J, Guan JF, Yao HQ, Jin XY (2016) Magnetic colorimetric immunoassay for human interleukin-6 based on the oxidase activity of ceria spheres. Anal Biochem 492:63-68. https://doi.org/10.1016/j.ab.2015.09.018
Lai WQ, Wei QH, Xu MD, Zhuang JY, Tang DP (2017) Enzyme-controlled dissolution of MnO2 nanoflakes with enzyme cascade amplification for colorimetric immunoassay. Biosens Bioelectron 89:645-651. https://doi.org/10.1016/j.bios.2015.12.035
Tan HL, Ma CJ, Gao L, Li Q, Song YH, Xu FG, Wang T, Wang L (2014) Metal-organic framework-derived copper nanoparticle@carbon nanocomposites as peroxidase mimics for colorimetric sensing of ascorbic acid. Chem-Eur J 20(49):16377-16383. https://doi.org/10.1002/chem.201404960
Dalapati R, Sakthivel B, Ghosalya MK, Dhakshinamoorthy A, Biswas S (2017) A cerium-based metal-organic framework having inherent oxidase-like activity applicable for colorimetric sensing of biothiols and aerobic oxidation of thiols. Crystengcomm 19(39):5915-5925. https://doi.org/10.1039/c7ce01053b
Liu YL, Fu WL, Li CM, Huang CZ, Li YF (2015) Gold nanoparticles immobilized on metal-organic frameworks with enhanced catalytic performance for DNA detection. Anal Chim Acta 861:55-61. https://doi.org/10.1016/j.aca.2014.12.032
Pham MH, Vuong T, Vu AT, Do TO (2011) Novel route to size-controlled Fe-MIL-88B-NH2 metal-organic framework nanocrystals. Langmuir 27(24):15261-15267. https://doi.org/10.1021/la203570h
Liu SF, Wang Y, Ming JJ, Lin Y, Cheng CB, Li F (2013) Enzyme-free and ultrasensitive electrochemical detection of nucleic acids by target catalyzed hairpin assembly followed with hybridization chain reaction. Biosens Bioelectron 49:472-477. https://doi.org/10.1016/j.bios.2013.05.037
Wen CY, Bi JH, Wu LL, Zeng JB (2018) Aptamer-functionalized magnetic and fluorescent nanospheres for one-step sensitive detection of thrombin. Microchim Acta 185(1):77. https://doi.org/10.1007/s00604-017-2621-5
Wang L, Yang W, Li TF, Li D, Cui ZM, Wang Y, Ji SL, Song QX, Shu C, Ding L (2017) Colorimetric determination of thrombin by exploiting a triple enzyme-mimetic activity and dual-aptamer strategy. Microchim Acta 184(9):3145-3151. https://doi.org/10.1007/s00604-017-2327-8
Wen DX, He MH, Ma KF, Cui Y, Kong JM, Yang HX, Liu QY (2018) Highly sensitive fluorometric determination of thrombin by on-chip signal amplification initiated by terminal deoxynucleotidyl transferase. Microchim Acta 185(8):380. https://doi.org/10.1007/S00604-018-2903-6
Higuchi A, Siao YD, Yang ST, Hsieh PV, Fukushima H, Chang Y, Ruaan RC, Chen WY (2008) Preparation of a DNA aptamer-Pt complex and its use in the colorimetric sensing of thrombin and anti-thrombin antibodies. Anal Chem 80(17):6580-6586. https://doi.org/10.1021/ac8006957
Liu YY, Zhao YH, Fan Q, Khan MS, Li XJ, Zhang Y, Ma HM, Wei Q (2018) Aptamer based electrochemiluminescent thrombin assay using carbon dots anchored onto silver-decorated polydopamine nanospheres. Microchim Acta 185(2):85. https://doi.org/10.1007/s00604-017-2616-2
Shi K, Dou BT, Yang JM, Yuan R, Xiang Y (2017) Target-triggered catalytic hairpin assembly and TdT-catalyzed DNA polymerization for amplified electronic detection of thrombin in human serums. Biosens Bioelectron 87:495-500. https://doi.org/10.1016/j.bios.2016.08.056
He BS (2018) Sandwich electrochemical thrombin assay using a glassy carbon electrode modified with nitrogen- and sulfur-doped graphene oxide and gold nanoparticles. Microchim Acta 185(7):344. https://doi.org/10.1007/S00604-018-2872-9
Fan TT, Du Y, Yao Y, Wu J, Meng S, Luo JJ, Zhang X, Yang DZ, Wang CY, Qian Y, Gao FL (2018) Rolling circle amplification triggered poly adenine-gold nanoparticles production for label-free electrochemical detection of thrombin. Sensor Actuat B-Chem 266:9-18. https://doi.org/10.1016/j.snb.2018.03.112
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.81373599), a National Major Difficult Disease Clinical Service Capacity Building project (No.2100202), and Special Projects of the National Chinese Medicine Industry (201507001-12).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 2033 kb)
Rights and permissions
About this article
Cite this article
Cheng, T., Li, X., Huang, P. et al. Colorimetric and electrochemical (dual) thrombin assay based on the use of a platinum nanoparticle modified metal-organic framework (type Fe-MIL-88) acting as a peroxidase mimic. Microchim Acta 186, 94 (2019). https://doi.org/10.1007/s00604-018-3209-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3209-4