Abstract
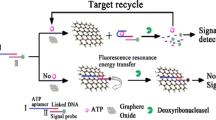
A fluorometric ATP assay is described that makes use of carbon dots and graphene oxide along with toehold-mediated strand displacement reaction. In the absence of target, the fluorescence of carbon dots (with excitation/emission maxima at 360/447 nm) is strong and in the “on” state, because the signal probe hybridizes with the aptamer strand and cannot combine with graphene oxide. In the presence of ATP, it will bind to the aptamer and induce a strand displacement reaction. Consequently, the signal probe is released, the sensing strategy will change into the “off” state with the addition of graphene oxide. This aptasensor exhibits selective and sensitive response to ATP and has a 3.3 nM detection limit.

Schematic of signal amplification by strand displacement in a carbon dot based fluorometric assay for ATP. This strategy exhibits high sensitivity and selectivity with a detection limit as low as 3.3 nM.





Similar content being viewed by others
References
Xu M, Gao Z, Zhou Q, Lin Y, Lu M, Tang D (2016) Terbium ion-coordinated carbon dots for fluorescent aptasensing of adenosine 5′-triphosphate with unmodified gold nanoparticles. Biosens Bioelectron 86:978–984. https://doi.org/10.1016/j.bios.2016.07.105
Kato M, Shiode N, Teragawa H, Hirao H, Yamada T (1999) Adenosine 5′-triphosphate induced dilation of human coronary microvessels in vivo. Intern Med 38:324–329. https://doi.org/10.2169/internalmedicine.38.324
Shinozaki Y, Koizumi S, Ishida S, Sawada J, Ohno Y, Inoue K (2005) Cytoprotection against oxidative stress-induced damage of astrocytes by extracellular ATP via P2Y1 receptors. Glia 49(2):288–300. https://doi.org/10.1002/glia.20118
Zhao Q, Zhang Z, Tang Y (2017) A new conjugated polymer-based combination probe for ATP detection using a multisite-binding and FRET strategy. Chem Commun 53:9414–9417. https://doi.org/10.1039/c7cc04293k
Hai X, Li N, Wang K, Zhang Z, Zhang J, Dang F (2018) A fluorescence aptasensor based on two-dimensional sheet metal-organic frameworks for monitoring adenosine triphosphate. Anal Chim Acta 998:60–66. https://doi.org/10.1016/j.aca.2017.10.028
Jiang G, Zhu W, Shen X, Xu L, Li X, Wang R, Liu C, Zhou X (2017) Colorimetric and visual determination of adenosine triphosphate using a boronic acid as the recognition element, and based on the deaggregation of gold nanoparticles. Microchim Acta 184(11):4305–4312. https://doi.org/10.1007/s00604-017-2454-2
Mao Y, Fan T, Gysbers R, Tan Y, Liu F (2017) A simple and sensitive aptasensor for colorimetric detection of adenosine triphosphate based on unmodified gold nanoparticles. Talanta 168:279–285. https://doi.org/10.1016/j.talanta.2017.03.014
Branchini BR, Southworth TL, Fontaine DM, Kohrt D, Talukder M, Michelini E, Cevenini L, Roda A, Grossel MJ (2015) An enhanced chimeric firefly luciferase-inspired enzyme for ATP detection and bioluminescence reporter and imaging applications. Anal Biochem 484:148–153. https://doi.org/10.1016/j.ab.2015.05.020
Shi F, Li Y, Lin Z, Ma D, Su X (2015) A novel fluorescent probe for adenosine 5′-triphosphate detection based on Zn2+−modulated l-cysteine capped CdTe quantum dots. Sensors Actuators B Chem 220:433–440. https://doi.org/10.1016/j.snb.2015.05.087
Shen X, Xu L, Zhu W, Li B, Hong J, Zhou X (2017) A turn-on fluorescence aptasensor based on carbon dots for sensitive detection of adenosine. New J Chem 41(17):9230–9235. https://doi.org/10.1039/C7NJ02384G
Zhu W, Shen X, Zhu C, Li B, Hong J, Zhou X (2018) Turn-on fluorescent assay based on purification system via magnetic separation for highly sensitive probing of adenosine. Sensors Actuators B Chem 259:855–861. https://doi.org/10.1016/j.snb.2017.12.147
Wang X, Shen X, Li B, Jiang G, Zhou X, Jiang H (2016) One-step facile synthesis of novel β-amino alcohol functionalized carbon dots for the fabrication of a selective copper ion sensing interface based on the biuret reaction. RSC Adv 6(22):18326–18332. https://doi.org/10.1039/C5RA24348C
Zuo P, Lu X, Sun Z, Guo Y, He H (2016) A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim Acta 183(2):519–542. https://doi.org/10.1007/s00604-015-1705-3
Dhenadhayalan N, Lin KC (2015) Chemically induced fluorescence switching of carbon-dots and its multiple logic gate implementation. Sci Rep 5:10012–10021. https://doi.org/10.1038/srep10012
Song Q, Peng M, Wang L, He D, Ouyang J (2016) A fluorescent aptasensor for amplified label-free detection of adenosine triphosphate based on core-shell Ag@SiO2 nanoparticles. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2015.09.008
Duan W, Wang X, Wang H, Li F (2018) Fluorescent and colorimetric dual-mode aptasensor for thrombin detection based on target-induced conjunction of split aptamer fragments. Talanta 180:76–80. https://doi.org/10.1016/j.talanta.2017.12.033
Sun C, Sun R, Chen Y, Tong Y, Zhu J, Bai H, Zhang S, Zheng H, Ye H (2018) Utilization of aptamer-functionalized magnetic beads for highly accurate fluorescent detection of mercury (II) in environment and food. Sensors Actuators B Chem 255:775–780. https://doi.org/10.1016/j.snb.2017.08.004
Liu Y, Liu C, Liu Y (2011) Investigation on fluorescence quenching of dyes by graphite oxide and graphene. Appl Surf Sci 257(13):5513–5518. https://doi.org/10.1016/j.apsusc.2010.12.136
Cheng X, Cen Y, Xu G, Wei F, Shi M, Xu X, Sohail M, Hu Q (2018) Aptamer based fluorometric determination of ATP by exploiting the FRET between carbon dots and graphene oxide. Microchim Acta 185(2). https://doi.org/10.1007/s00604-018-2683-z
Ning Y, Wei K, Cheng L, Hu J, Xiang Q (2017) Fluorometric aptamer based determination of adenosine triphosphate based on deoxyribonuclease I-aided target recycling and signal amplification using graphene oxide as a quencher. Microchim Acta 184(6):1847–1854. https://doi.org/10.1007/s00604-017-2194-3
Zhu W, Zhao Z, Li Z, Li H, Jiang J, Shen G, Yu R (2013) A label free exonuclease III-aided fluorescence assay for adenosine triphosphate based on graphene oxide and ligation reaction. New J Chem 37(4):927. https://doi.org/10.1039/c2nj41055a
Wen C, Huang Y, Tian J, Hu K, Pan L, Zhao S (2015) A novel exonuclease III-aided amplification assay based on a graphene platform for sensitive detection of adenosine triphosphate. Anal Methods-Uk 7(9):3708–3713. https://doi.org/10.1039/C5AY00354G
Hong F, Chen X, Cao Y, Dong Y, Wu D, Hu F, Gan N (2018) Enzyme- and label-free electrochemical aptasensor for kanamycin detection based on double stir bar-assisted toehold-mediated strand displacement reaction for dual-signal amplification. Biosens Bioelectron 112:202–208. https://doi.org/10.1016/j.bios.2018.04.017
Chen J, Shang B, Zhang H, Zhu Z, Chen L, Wang H, Ran F, Chen Q, Chen J (2018) Enzyme-free ultrasensitive fluorescence detection of epithelial cell adhesion molecules based on a toehold-aided DNA recycling amplification strategy. RSC Adv 8(27):14798–14805. https://doi.org/10.1039/C8RA01362D
Lv Y, Cui L, Peng R, Zhao Z, Qiu L, Chen H, Jin C, Zhang X, Tan W (2015) Entropy beacon: a hairpin-free DNA amplification strategy for efficient detection of nucleic acids. Anal Chem 87(23):11714–11720. https://doi.org/10.1021/acs.analchem.5b02654
Zhang DY, Winfree E (2009) Control of DNA strand displacement kinetics using toehold exchange. J Am Chem Soc 131(47):17303–17314. https://doi.org/10.1021/ja906987s
He J, Zhang H, Zou J, Liu Y, Zhuang J, Xiao Y, Lei B (2016) Carbon dots-based fluorescent probe for "off-on" sensing of hg(II) and I−. Biosens Bioelectron 79:531–535. https://doi.org/10.1016/j.bios.2015.12.084
H JWS, E OR (1958) Preparation of graphitic oxide. J Am Chem Soc 80(6):1339
Cui X, Wang Y, Liu J, Yang Q, Zhang B, Gao Y, Wang Y, Lu G (2017) Dual functional N- and S-co-doped carbon dots as the sensor for temperature and Fe3+ ions. Sensors Actuators B Chem 242:1272–1280. https://doi.org/10.1016/j.snb.2016.09.032
Wu ZL, Gao MX, Wang TT, Wan XY, Zheng LL (2014) A general quantitative pH sensor developed with dicyandiamide N-doped high quantum yield graphene quantum dots. Nanoscale 6:3868–3874. https://doi.org/10.1039/c3nr06353d
Zhai X, Zhang P, Liu C, Bai T, Li W, Dai L, Liu W (2012) Highly luminescent carbon nanodots by microwave-assisted pyrolysis. Chem Commun 48(64):7955. https://doi.org/10.1039/c2cc33869f
Wang P, Cheng Z, Chen Q, Qu L, Miao X, Feng Q (2018) Construction of a paper-based electrochemical biosensing platform for rapid and accurate detection of adenosine triphosphate (ATP). Sensors Actuators B Chem 256:931–937. https://doi.org/10.1016/j.snb.2017.10.024
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81773681, 81572081, 81273480, and 21175070).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 1288 kb)
Rights and permissions
About this article
Cite this article
Luo, J., Shen, X., Li, B. et al. Signal amplification by strand displacement in a carbon dot based fluorometric assay for ATP. Microchim Acta 185, 392 (2018). https://doi.org/10.1007/s00604-018-2931-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2931-2