Abstract
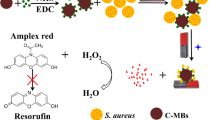
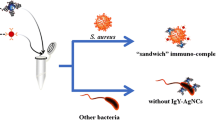
An affinity-based protocol is described for the detection of Staphylococcus aureus (S. aureus). It is utilizing teicoplanin-functionalized magnetic beads as carriers. Teicoplanin, which binds to the walls of cells of S. aureus via five hydrogen bonds, acts as the recognition agent. Captured S. aureus is magnetically separated from the sample matrix and then specifically lysed by lysostaphin which cleaves the cross-linking pentaglycine bridges of peptidoglycan in the cell wall. Lastly, S. aureus is quantified via the inhibitory effect of released intracellular catalase on a chemiluminescent (CL) system composed of peroxidase, luminol, H2O2 and p-iodophenol because catalase decomposes H2O2. S. aureus can be detected with CL response in the 140 to 1.4 × 107 CFU·mL−1 concentration range and a detection limit as low as 47 CFU·mL−1 at a signal-to-noise ratio of 3. The method was evaluated by analyzing spiked samples including milk, human urine and saline injection solutions. The reliability was demonstrated by a recovery test and by comparison with a conventional plate counting method.

An antibiotic-affinity protocol is developed to detect Staphylococcus aureus (S. aureus) by utilizing teicoplanin-functionalized magnetic beads (Teic-MBs) as carriers. S. aureus can be quantified by measuring the inhibition of luminol chemiluminescence (CL) signal by intracellular catalase.




Similar content being viewed by others
References
Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Dopfer D, Fazil A, Fischer-Walker CL, Hald T, Hall AJ, Keddy KH, Lake RJ, Lanata CF, Torgerson PR, Havelaar AH, Angulo FJ (2015) World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med 12(12):e1001921. https://doi.org/10.1371/journal.pmed.1001921
Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339(8):520–532. https://doi.org/10.1056/nejm199808203390806
Law JWF, Ab Mutalib NS, Chan KG, Lee LH (2014) Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front Microbiol 5(770):1–19. https://doi.org/10.3389/fmicb.2014.00770
Zhao X, Lin CW, Wang J, Oh DH (2014) Advances in rapid detection methods for foodborne pathogens. J Microbiol Biotechnol 24(3):297–312. https://doi.org/10.4014/jmb.1310.10013
Dou M, Sanjay ST, Dominguez DC, Liu P, Xu F, Li X (2017) Multiplexed instrument-free meningitis diagnosis on a polymer/paper hybrid microfluidic biochip. Biosens Bioelectron 87:865–873. https://doi.org/10.1016/j.bios.2016.09.033
Ma K, Deng Y, Bai Y, Xu DX, Chen EN, Wu HJ, Li BM, Gao LJ (2014) Rapid and simultaneous detection of Salmonella, Shigella, and Staphylococcus aureus in fresh pork using a multiplex real-time PCR assay based on immunomagnetic separation. Food Control 42:87–93. https://doi.org/10.1016/j.foodcont.2014.01.042
Park SH, Aydin M, Khatiwara A, Dolan MC, Gilmore DF, Bouldin JL, Ahn S, Ricke SC (2014) Current and emerging technologies for rapid detection and characterization of Salmonella in poultry and poultry products. Food Microbiol 38:250–262. https://doi.org/10.1016/j.fm.2013.10.002
Sung YJ, Suk HJ, Sung HY, Li T, Poo H, Kim MG (2013) Novel antibody/gold nanoparticle/magnetic nanoparticle nanocomposites for immunomagnetic separation and rapid colorimetric detection of Staphylococcus aureus in milk. Biosens Bioelectron 43:432–439. https://doi.org/10.1016/j.bios.2012.12.052
Tan F, Leung PHM, Z-b L, Zhang Y, Xiao L, Ye W, Zhang X, Yi L, Yang M (2011) A PDMS microfluidic impedance immunosensor for E. coli O157:H7 and Staphylococcus aureus detection via antibody-immobilized nanoporous membrane. Sens Actuators B Chem 159(1):328–335. https://doi.org/10.1016/j.snb.2011.06.074
Abbaspour A, Norouz-Sarvestani F, Noori A, Soltani N (2015) Aptamer-conjugated silver nanoparticles for electrochemical dual-aptamer-based sandwich detection of Staphylococcus aureus. Biosens Bioelectron 68:149–155. https://doi.org/10.1016/j.bios.2014.12.040
Duan N, Wu SJ, Zhu CQ, Ma XY, Wang ZP, Yu Y, Jiang Y (2012) Dual-color upconversion fluorescence and aptamer-functionalized magnetic nanoparticles-based bioassay for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus. Anal Chim Acta 723:1–6. https://doi.org/10.1016/j.aca.2012.02.011
Akram AR, Avlonitis N, Lilienkampf A, Perez-Lopez AM, McDonald N, Chankeshwara SV, Scholefield E, Haslett C, Bradley M, Dhaliwal K (2015) A labelled-ubiquicidin antimicrobial peptide for immediate in situ optical detection of live bacteria in human alveolar lung tissue. Chem Sci 6(12):6971–6979. https://doi.org/10.1039/c5sc00960j
Liu XB, Marrakchi M, Xu DW, Dong H, Andreescu S (2016) Biosensors based on modularly designed synthetic peptides for recognition, detection and live/dead differentiation of pathogenic bacteria. Biosens Bioelectron 80:9–16. https://doi.org/10.1016/j.bios.2016.01.041
Liu P, Han L, Wang F, Petrenko VA, Liu AH (2016) Gold nanoprobe functionalized with specific fusion protein selection from phage display and its application in rapid, selective and sensitive colorimetric biosensing of Staphylococcus aureus. Biosens Bioelectron 82:195–203. https://doi.org/10.1016/j.bios.2016.03.075
Rees JC, Pierce CL, Schieltz DM, Barr JR (2015) Simultaneous identification and susceptibility determination to multiple antibiotics of Staphylococcus aureus by bacteriophage amplification detection combined with mass spectrometry. Anal Chem 87(13):6769–6777. https://doi.org/10.1021/acs.analchem.5b00959
Williams DH (1996) The glycopeptide story - how to kill the deadly 'superbugs. Nat Prod Rep 13(6):469–477. https://doi.org/10.1039/NP9961300469
Reynolds PE (1989) Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur J Clin Microbiol Infect Dis 8(11):943–950. https://doi.org/10.1007/bf01967563
Browder HP, Zygmunt WA, Young JR, Tavormin PA (1965) Lysostaphin - enzymatic mode of action. Biochem Biophys Res Commun 19(3):383–389. https://doi.org/10.1016/0006-291x(65)90473-0
Chelikani P, Fita I, Loewen PC (2004) Diversity of structures and properties among catalases. Cell Mol Life Sci 61(2):192–208. https://doi.org/10.1007/s00018-003-3206-5
Wu JA, Kusuma C, Mond JJ, Kokai-Kun JF (2003) Lysostaphin disrupts Staphylococcus aureus and Staphylococcus epidermidis biofilms on artificial surfaces. Antimicrob Agents Chemother 47(11):3407–3414. https://doi.org/10.1128/aac.47.11.3407-3414.2003
Acknowledgements
This work was financially supported by the Natural Science Foundation of China (21475107) and the Fundamental Research Funds for the Central Universities (XDJK2017A008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 74.3 kb)
Rights and permissions
About this article
Cite this article
Wu, Y., Wang, M., Ouyang, H. et al. Teicoplanin-functionalized magnetic beads for detection of Staphylococcus aureus via inhibition of the luminol chemiluminescence by intracellular catalase. Microchim Acta 185, 391 (2018). https://doi.org/10.1007/s00604-018-2921-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2921-4