Abstract
Magnesium(II)-doped nickel ferrite (Mg–NiFe2O4) nanoparticles are introduced as a new adsorbent for magnetic solid phase extraction of lead(II) ions from aqueous solutions. The structure and morphology of the adsorbent was characterized by FTIR, X-ray diffraction and scanning electron microscopy. The effects of pH value, amount of adsorbent, type, concentration and volume of the eluent and adsorption/desorption time on the extraction efficiency were studied. Following elution with hydrochloric acid, Pb(II) ions were quantified by flame atomic absorption spectrometry. Under optimized conditions, the calibration graph is linear in the 0.5–125 ng mL−1 Pb(II) ion concentration range. Other figures of merit include (a) a 0.2 ng mL−1 limit of detection, (b) an enrichment factor of 200, (c) an intra-day relative standard deviation (for n = 6 at 50 ng mL−1) of 1.6%, and (d) an inter-day precision of 3.8%. The method was validated by the analysis of the certified reference material, NIST SRM 1566b. It was successfully applied to the determination of Pb(II) ion in spiked water samples, industrial wastewater and acidic lead battery waters.

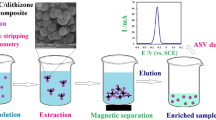
Schematic of the synthesis of Mg(II)-doped NiFeO4 nanoparticles and their application as a magnetic sorbent for solid-phase extraction of a Pb(II) ions prior to determination by flame atomic absorption spectrometry (FAAS).




Similar content being viewed by others
References
Shah F, Kazi TG, Afridi HI, Soylak M (2012) Temperature controlled ionic liquid–dispersive liquid phase microextraction for determination of trace lead level in blood samples prior to analysis by flame atomic absorption spectrometry with multivariate optimization. Microchem J 101:5–10
Hameed SM, Al–Kubaisy RK, Hussain KI (2015) Liquid ion exchange method for extraction of Pb (II) as anion by using 15C5 coupled with spectrophotometric determination in different samples. J Kufa Chem Sci 1:21–37
Vilar VJ, Sebesta F, Botelho CM, Boaventura RA (2005) Equilibrium and kinetic modelling of Pb 2+ biosorption by granulated agar extraction algal waste. Process Biochem 40:3276–3284
Qian ZS, Shan XY, Chai LJ, Chen JR, Feng H (2015) A fluorescent nanosensor based on graphene quantum dots–aptamer probe and graphene oxide platform for detection of lead (II) ion. Biosens Bioelectron 68:225–231
Zhang C, Zhou Y, Tang L, Zeng G, Zhang J, Peng B, Xie X, Lai C, Long B, Zhu J (2016) Determination of Cd2+ and Pb2+ based on mesoporous carbon nitride/self–doped polyaniline nanofibers and square wave anodic stripping voltammetry. Nanomaterials 6:7–17
Golshekan M, Shariati S (2013) Nano magnetic solid phase extraction for preconcentration of lead ions in environmental samples by a newly synthesized reagent. Acta Chim Slov 60:358–367
Ahmad R, Kumar R, Laskar MA (2013) Adsorptive removal of Pb2+ form aqueous solution by macrocyclic calix naphthalene: kinetic, thermodynamic, and isotherm analysis. Environ Sci Pollut Res 20:219–226
Shi Y, Wang H, Jiang X, Sun B, Song B, Su Y, He Y (2016) Ultrasensitive, specific, recyclable, and reproducible detection of lead ions in real systems through a polyadenine–assisted, surface–enhanced Raman scattering silicon chip. Anal Chem 88:3723–3729
Koohi F, Shokrollahi A (2015) Determination of Pb2+ ion in water samples by CPE–FAAS using erythrosine as complexing agent. Int J ChemTech Res 8:227–222
Dias LF, Saint'Pierre TD, Maia SM, Silva MAM, Frescura VL, Welz B, Curtius AJ (2002) Determination of arsenic, lead, selenium and tin in sediments by slurry sampling electrothermal vaporization inductively coupled plasma mass spectrometry using Ru as permanent modifier and NaCl as a carrier. Spectrochim Acta B At Spectrosc 57:2003–2015
Guzmán–Mar JL, Hinojosa–Reyes L, Serra AM, Hernández–Ramírez A, Cerdà V (2011) Applicability of multisyringe chromatography coupled to cold–vapor atomic fluorescence spectrometry for mercury speciation analysis. Anal Chim Acta 708:11–18
Arpadjan S, Celik G, Taşkesen S, Güçer Ş (2008) Arsenic, cadmium and lead in medicinal herbs and their fractionation. Food Chem Toxicol 46:2871–2875
Ghaedi M, Karimipour G, Alambarkat E, Asfaram A, Montazerozohor M, Izadpanah S, Soylak M (2015) Solid–phase extraction of Pb2+ ion from environmental samples onto L–AC–Ag–NP by flame atomic absorption spectrometry (FAAS). Int J Environ Anal Chem 95:1030–1041
Emadi M, Shams E (2013) Preconcentration of Pb2+ by iron oxide/amino–functionalized silica core–shell magnetic nanoparticles as a novel solid–phase extraction adsorbent and its determination by flame atomic absorption spectrometry. J Iran Chem Soc 10:325–332
Ghaedi M, Shokrollahi A, Niknam K, Niknam E, Najibi A, Soylak M (2009) Cloud point extraction and flame atomic absorption spectrometric determination of cadmium (II), lead (II), palladium (II) and silver (I) in environmental samples. J Hazard Mater 168:1022–1027
Jafari Pirouz M, Hossein Beyki M, Shemirani F (2015) Anhydride functionalised calcium ferrite nanoparticles: a new selective magnetic material for enrichment of lead ions from water and food samples. Food Chem 170:131–137
Abdolmohammad-Zadeh H, Rahimpour E (2015) A novel chemosensor for Ag (I) ion based on its inhibitory effect on the luminol–H2O2 chemiluminescence response improved by CoFe2O4 nano–particles. Sensors Actuators B Chem 209:496–504
Reddy DHK, Yun Y (2016) Spinel ferrite magnetic adsorbents: alternative future materials for water purification. Coord Chem Rev 315:90–111
Zandipak R, Sobhanardakani S (2016) Synthesis of NiFe2O4 nanoparticles for removal of anionic dyes from aqueous solution. Desalin Water Treat 57:11348–11360
Moradmard H, Shayesteh SF, Tohidi P, Abbas Z, Khaleghi M (2015) Structural, magnetic and dielectric properties of magnesium doped nickel ferrite nanoparticles. J Alloys Compd 650:116–122
Abdolmohammad-Zadeh H, Rahimpour E (2016) A novel chemosensor based on graphitic carbon nitride quantum dots and potassium ferricyanide chemiluminescence system for Hg (II) ion detection. Sensors Actuators B Chem 225:258–266
Gabal MA, Al Angari YM, Zaki HM (2014) Structural, magnetic and electrical characterization of Mg–Ni nano–crystalline ferrites prepared through egg–white precursor. J Magn Magn Mater 363:6–12
Reddy DHK, Lee SM (2013) Three-dimensional porous spinel ferrite as an adsorbent for Pb(II) removal from aqueous solutions. Ind Eng Chem Res 52:15789–15800
Yavuz E, Tokalıoğlu Ş, Şahan H, Patat Ş (2016) Nanosized spongelike Mn3O4 as an adsorbent for preconcentration by vortex assisted solid phase extraction of copper and lead in various food and herb samples. Food Chem 194:463–469
Barciela-Alonso MC, Plata-García V, Rouco-López A, Moreda-Piñeiro A, Bermejo-Barrera P (2014) Ionic imprinted polymer based solid phase extraction for cadmium and lead pre-concentration/determination in seafood. Microchem J 114:106–110
Karimi M, Shabani AMH, Dadfarnia S (2016) Deep eutectic solvent-mediated extraction for ligand-less preconcentration of lead and cadmium from environmental samples using magnetic nanoparticles. Microchim Acta 183:563–571
Zawisza B, Baranik A, Malicka E, Talik E, Sitko R (2016) Preconcentration of Fe(III), Co(II), Ni(II), Cu(II), Zn(II) and Pb(II) with ethylenediamine-modified graphene oxide. Microchim Acta 183:231–240
Behbahani M, Bide Y, Bagheri S, Salarian M, Omidi F, Nabid MR (2016) A pH responsive nanogel composed of magnetite, silica and poly(4-vinylpyridine) for extraction of Cd(II), Cu(II), Ni(II) and Pb(II). Microchim Acta 183:111–121
Asiabi M, Mehdinia A, Jabbari A (2017) Spider-web-like chitosan/MIL-68(Al) composite nanofibers for high-efficient solid phase extraction of Pb(II) and Cd(II). Microchim Acta 18:4495–4501
Baghban N, Yilmaz E, Soylak M (2017) A magnetic MoS2-Fe3O4 nanocomposite as an effective adsorbent for dispersive solid-phase microextraction of lead(II) and copper(II) prior to their determination by FAAS. Microchim Acta 184:3969–3976
Habila MA, ALOthman ZA, El-Toni AM, Al-Tamrah SA, Soylak M, Labis JP (2017) Carbon-coated Fe3O4 nanoparticles with surface amido groups for magnetic solid phase extraction of Cr(III), Co(II), Cd(II), Zn(II) and Pb(II) prior to their quantitation by ICP-MS. Microchim Acta 184:2645–2651
Mehdinia A, Shoormeij Z, Jabbari A (2017) Trace determination of lead(II) ions by using a magnetic nanocomposite of the type Fe3O4/TiO2/PPy as a sorbent, and FAAS for quantitation. Microchim Acta 184:1529–1537
Ghorbani-Kalhor E (2016) A metal-organic framework nanocomposite made from functionalized magnetite nanoparticles and HKUST-1 (MOF-199) for preconcentration of Cd(II), Pb(II), and Ni(II). Microchim Acta 183:2639–2647
Aghagoli MJ, Shemirani F (2017) Hybrid nanosheets composed of molybdenum disulfide and reduced graphene oxide for enhanced solid phase extraction of Pb(II) and Ni(II). Microchim Acta 184:237–244
Fahimirad B, Asghari A, Rajabi M (2017) Magnetic graphitic carbon nitride nanoparticles covalently modified with an ethylenediamine for dispersive solid-phase extraction of lead(II) and cadmium(II) prior to their quantitation by FAAS. Microchim Acta 184:3027–3035
Acknowledgments
This work was supported by the research council of Azarbaijan Shahid Madani University (Grant no. ASMU/96372-17).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 112 kb)
Rights and permissions
About this article
Cite this article
Abdolmohammad-Zadeh, H., Salimi, A. Preconcentration of Pb(II) by using Mg(II)-doped NiFe2O4 nanoparticles as a magnetic solid phase extraction agent. Microchim Acta 185, 343 (2018). https://doi.org/10.1007/s00604-018-2874-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2874-7