Abstract
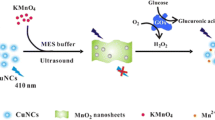
The authors report that carbon nitride quantum dots (CN QDs) exert a strong enhancing effect on the Cu(II)/H2O2 chemiluminescent system. Chemiluminescence (CL) intensity is enhanced by CN QDs by a factor of ~75, while other carbon nanomaterials have a much weaker effect. The possible mechanism of the effect was evaluated by recording fluorescence and CL spectra and by examining the effect of various radical scavengers. Emitting species was found to be excited-state CN QDs that produce green CL peaking at 515 nm. The new CL system was applied to the sensitive detection of H2O2 and glucose (via glucose oxidase-catalyzed formation of H2O2) with detection limits (3σ) of 10 nM for H2O2 and 100 nM for glucose. The probe was employed for glucose determination in human plasma samples with satisfactory results.

The effect of carbon nitride quantum dots (CN QDs) on Cu(II)-H2O2 chemiluminescence reaction was studied and the new CL system was applied for sensitive detection of glucose based on the glucose oxidase (GOx)-catalyzed formation of H2O2.





Similar content being viewed by others
References
Bubb KJ, Birgisdottir AB, Tang O et al (2017) Redox modification of caveolar proteins in the cardiovascular system- role in cellular signalling and disease. Free Radic Biol Med 109:61–74
Liu H, Weng L, Yang C (2017) A review on nanomaterial-based electrochemical sensors for H2O2, H2S and NO inside cells or released by cells. Microchim Acta 184:1267–1283
Chen X, Wu G, Cai Z et al (2014) Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim Acta 181:689–705
Ma Z-Z, H-C Y, Z-Y W et al (2016) A highly sensitive amperometric glucose biosensor based on a nano-cube Cu2O modified glassy carbon electrode. Chin J Anal Chem 44:822–827
Hu Y, Cheng H, Zhao X et al (2017) Surface-enhanced raman scattering active gold nanoparticles with enzyme-mimicking activities for measuring glucose and lactate in living tissues. ACS Nano 11:5558–5566
Wei H, Wang E (2008) Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal Chem 80:2250–2254
Guo C, Song Y, Wei H et al (2007) Room temperature ionic liquid doped DNA network immobilized horseradish peroxidase biosensor for amperometric determination of hydrogen peroxide. Anal Bioanal Chem 389:527–532
Lin Z, Chen H, Lin J-M (2013) Peroxide induced ultra-weak chemiluminescence and its application in analytical chemistry. Analyst 138:5182
Hanaoka S, Lin J-M, Yamada M (2000) Chemiluminescence behavior of the decomposition of hydrogen peroxide catalyzed by copper(II)–amino acid complexes and its application to the determination of tryptophan and phenylalanine. Anal Chim Acta 409:65–73
Liu M, Li B, Zhang Z, Lin J-M (2005) Enhancing effect of DNA on chemiluminescence from the decomposition of hydrogen peroxide catalyzed by copper(II). Anal Bioanal Chem 381:828–832
Zhang L, Zhang Z, Lu C, Lin J-M (2012) Improved chemiluminescence in Fenton-like reaction via dodecylbenzene-sulfonate-intercalated layered double hydroxides. J Phys Chem C 116:14711–14716
Shi J, Lu C, Yan D, Ma L (2013) High selectivity sensing of cobalt in HepG2 cells based on necklace model microenvironment-modulated carbon dot-improved chemiluminescence in Fenton-like system. Biosens Bioelectron 45:58–64
Pan F, Wei P, Zhang M, Lu C (2015) Micelle modified-carbon nanosphere enhanced chemiluminescence from reactive oxygen species for the detection of hydrogen peroxide. Anal Methods 7:5667–5673
Liu J, Wang H, Antonietti M (2016) Graphitic carbon nitride “reloaded”: emerging applications beyond (photo)catalysis. Chem Soc Rev 45:2308–2326
Wang A-J, Li H, Huang H et al (2016) Fluorescent graphene-like carbon nitrides: synthesis, properties and applications. J Mater Chem C 4:8146–8160
Tang Y, Su Y, Yang N et al (2014) Carbon nitride quantum dots: a novel chemiluminescence system for selective detection of free chlorine in water. Anal Chem 86:4528–4535
Fan X, Su Y, Deng D, Lv Y (2016) Carbon nitride quantum dot-based chemiluminescence resonance energy transfer for iodide ion sensing. RSC Adv 6:76890–76896
Fan X, Feng Y, Su Y et al (2015) A green solid-phase method for preparation of carbon nitride quantum dots and their applications in chemiluminescent dopamine sensing. RSC Adv 5:55158–55164
Yu H, He Y, Li W, Duan T (2015) Graphitic carbon nitride nanosheets-enhanced chemiluminescence of luminol for sensitive detection of 2,4,6-trinitrotoluene. Sens Actuators B Chem 220:516–521
Zhou J, Yang Y, Zhang C (2013) A low-temperature solid-phase method to synthesize highly fluorescent carbon nitride dots with tunable emission. Chem Commun 49:8605
Amjadi M, Manzoori JL, Hallaj T, Sorouraddin MH (2014) Direct chemiluminescence of carbon dots induced by potassium ferricyanide and its analytical application. Spectrochim Acta A Mol Biomol Spectrosc 122:715–720
Amjadi M, Hallaj T, Asadollahi H et al (2017) Facile synthesis of carbon quantum dot/silver nanocomposite and its application for colorimetric detection of methimazole. Sens Actuators B Chem 244:425–432
Amjadi M, Manzoori JL, Hallaj T, Shahbazsaghir T (2017) Application of the chemiluminescence system composed of silicon-doped carbon dots, iron(II) and K2S2O8 to the determination of norfloxacin. Microchim Acta 184:1587–1593
Chandra S, Laha D, Pramanik A et al (2016) Synthesis of highly fluorescent nitrogen and phosphorus doped carbon dots for the detection of Fe3+ ions in cancer cells: synthesis of highly fluorescent N and P doped carbon dots. Luminescence 31:81–87
Amjadi M, Manzoori JL, Hallaj T (2014) Chemiluminescence of graphene quantum dots and its application to the determination of uric acid. J Lumin 153:73–78
Cui Y, Ding Z, Liu P et al (2012) Metal-free activation of H2O2 by g-C3N4 under visible light irradiation for the degradation of organic pollutants. Phys Chem Chem Phys 14:1455–1462
Ju E, Dong K, Chen Z et al (2016) Copper(II)-graphitic carbon nitride triggered synergy: improved ROS generation and reduced glutathione levels for enhanced photodynamic therapy. Angew Chem 128:11639–11643
Wang W, Schuchmann MN, Schuchmann H-P et al (1999) Radical cations in the OH-radical-induced oxidation of thiourea and tetramethylthiourea in aqueous solution. J Am Chem Soc 121:238–245
Hosaka S, Itagaki T, Kuramitsu Y (1999) Selectivity and sensitivity in the measurement of reactive oxygen species (ROS) using chemiluminescent microspheres prepared by the binding of acridinium ester or ABEI to polymer microspheres. Luminescence 14:349–354
Chen YT, Zheng RL, Jia ZJ, Ju Y (1990) Flavonoids as superoxide scavengers and antioxidants. Free Radic Biol Med 9:19–21
Bagchi D, Garg A, Krohn RL et al (1997) Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res Commun Mol Pathol Pharmacol 95:179–189
Shah SNA, Lin L, Zheng Y et al (2017) Redox cycling of iron by carbon dot enhanced chemiluminescence: mechanism of electron–hole induction in carbon dot. Phys Chem Chem Phys 19:21604–21611
Jiang J, He Y, Li S, Cui H (2012) Amino acids as the source for producing carbon nanodots: microwave assisted one-step synthesis, intrinsic photoluminescence property and intense chemiluminescence enhancement. Chem Commun 48:9634
Amjadi M, Manzoori JL, Hallaj T (2015) A novel chemiluminescence method for determination of bisphenol abased on the carbon dot-enhanced HCO3 −–H2O2 system. J Lumin 158:160–164
Acknowledgments
This work was supported by a grant from Iran National Science Foundation (INSF 95848790).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 1040 kb)
Rights and permissions
About this article
Cite this article
Hallaj, T., Amjadi, M., Song, Z. et al. Strong enhancement of the chemiluminescence of the Cu(II)-H2O2 system on addition of carbon nitride quantum dots, and its application to the detection of H2O2 and glucose. Microchim Acta 185, 67 (2018). https://doi.org/10.1007/s00604-017-2547-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2547-y