Abstract
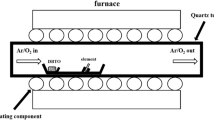
One dimensional nanostructured branched wires of tin dioxide (SnO2) were prepared in an alumina boat by a self catalytic method at different temperatures in a flow of argon and oxygen. The SEM images exhibit the different morphologies of the nanowires with an i.d. of around 40 nm, and presence of core/cluster shell nanowires and of nano/microbelt structures. XRD studies show the product to possess a typical tin dioxide nanowire structure. FTIR and Raman studies revealed the bond information. The sensing properties were studied by monitoring the changes in conductivity on exposure to hydrogen gas. Hydrogen can be detected in the 1000 ppm to 3000 ppm concentration range, and the limit of detection for H2 is 1000 ppm.

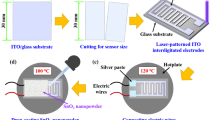
SnO2 nanostructures were prepared using chemical vapor deposition in an alumina boat, without substrate. The nanowires are coated on the overhead projector sheet. It is used to detect hydrogen gas via conductometry under flexible conditions.







Similar content being viewed by others
References
Jaya S, Gobinda Gopal K, Basmallick A (2007) Nanowires: properties, applications and synthesis via porous anodic aluminum oxide template. Bull. Mater. Sci 30:271–290
Nam SH, Boo JH (2012) Rutile structured SnO2 nanowires synthesized with metal catalyst by thermal evaporation method. J Nanosci Nanotech 12:1559–1562
Dai ZR, Gole JL, Stout JD, Wang ZL (2002) Tin oxide nanowires, nanoribbons, and nanotubes. J Phys Chem B 106:1274–1279
Zhou ZY, Pang H, Sun Y, Lai WY, Ang W, Huang W (2013) Porous tin oxide Nanoplatelets as excellent-efficiency photo electrodes and gas sensors. Int J Electrochem Sci 8:3371–3378
Yang R, Wang ZL (2006) Springs, rings, and spirals of rutile-structured tin oxide Nanobelt. J. Am. Chem. Soc 128(5):1466–1467
Masuda Y, Kato K (2012) SnO2 Nanosheet assembly coating on Teflon rods. J. Aust Ceram Soc 48(2):185–188
Cheng Y, Yang R, Zheng JP, Wang ZL, Xiong P (2012) Characterizing individual SnO2 nanobelt field-effect transistors and their intrinsic responses to hydrogen and ambient gases. Mater Chem Phys 137:372–380
Saravanan N, Ponnusamy S, Shri Prasad S, Joseph V (2014) Synthesis and characterization of SnO2 quantum dots. Int J Chem Tech Res 6(1):600–603
Shukla GP, Bhatnagar MC (2013) Effect of substrate on the morphology of SnO2 nanowire, Int J Innov Technol Exp Eng (IJITEE) 2(5):246–248.
Ristoscu C, Cultrera L, Dima A, Perrone A, Cutting R, Du HL, Busiakiewicz A, Klusek Z, Datta PK, Rose SR (2005) SnO2 nanostructured films obtained by pulsed laser ablation deposition. Appl Surf Sci 247:95–100
Tan L, Wang L, Wang Y (2011) Hydrothermal synthesis of SnO2 nanostructures with different morphologies and their optical properties. J Nanomater 2011:1–10
Kanazawa E, Sakai G, Shimanoe K, Kanmura Y, Teraoka Y, Miura N, Yamszoe N (2001) Metal oxide semiconductor N2O sensor for medical use, sensors and Actuators. B: Chem 77(1-2):72–77
Korotcenkov G, Brinzari V, Cho BK (2016) Conductometric gas sensors based on metal oxides modified with gold nanoparticles: a review. Microchim Acta 183:1033–1054
Brown WJ (1997) Safety standard for hydrogen and hydrogen systems. National Aeronautics and Space Administration (NASA), retrieved 5 February 2008:1–389
Tiong TY, Yahaya M, Dee CF, Lim KP, Majlis BY, Sow CH (2009) Influence of growth temperature on SnO2 nanowires. Mater Res Innov 13(3):203–206
Joharil A, Bhatnagar MC, Rana V (2012) Growth, characterization and I-V characteristics of tin oxide (SnO2) nanowires. Adv Mat Lett 3(6):515–518
Thabethe BS, Malgas GF, Motaung DE, Malwela T, Arendse CJ (2013) Self-catalytic growth of tin oxide nanowires by chemical vapor deposition process. J Nanomater 2013:1–7
Dahoudi NA (2011) Low temperature gas sensing coatings made through wet chemical deposition of niobium doped titanium oxide colloid. Mater Sci Appl 2:265–269
Roy S, Saha H, Sarkar CK (2010) High sensitivity methane sensor by chemically deposited nanocrystalline ZnO thin film. Int J Smart Sens Intell Syst 3(4):605–620
Sunghoon P, Soohyun K, Lee WI, Kim KK, Lee C (2014) Room temperature, ppb-level NO2 gas sensing of multiplenetworked ZnSe nanowire sensors under UV illumination. Beilstein J. Nanotechnol 5:1836–1841
Niu S, Hu Y, Wen X, Zhou Y, Zhang F, Lin L, Wang S, Wang ZL (2013) Enhanced performance of flexible ZnO nanowire based room-temperature oxygen sensors by Piezotronic effect. Adv. Mater 25(27):3701–3706
Zhan S, Li D, Liang S, Chen X, Li XA (2013) Novel flexible room temperature ethanol gas sensor based on SnO2 doped poly-diallyldimethylammonium chloride, sensors 2;13(4):4378-4389.
Acuautla M, Bernardini S, Bendahan M, Gallais L (2014) Ammonia sensing properties of ZnO nanoparticles on flexible substrate, proceedings of the 8th International conference on sensing Technology, 23-26.
Chen W, Gan H, Zhang W, Mao Z (2014) Hydrothermal synthesis and hydrogen sensing properties of nanostructured SnO2 with different morphologies. J Nanomater 2014:1–7
Ponnusamy D, Prasad AK, Madanagurusamy S (2016) CdO-TiO2 nanocomposite thin films for resistive hydrogen sensing. Microchim Acta 183:311–317
Hind IA, Hassan HD, Hassan FM (2016) Hydrogen gas sensor based on ZnO Nanoroads grown on Si by thermal evaporation. J Mater Sci Eng A 6(3–4):51–56
Chung MG, Kim DH, Seo DK, Kim T, Im HU, Lee HM, Yoo JB, Hong SH, Kang TJ, Kim YH (2012) Flexible hydrogen sensors using graphene with palladium nanoparticle decoration. Sens Actuators B 169:387–392
Lim SH, Radha B, Chan JY, Saifullah MSM, Kulkarni GU, Ho GW (2013) Flexible palladium-based H2 sensor with fast response and low leakage detection by nanoimprint lithography. ACS Appl Mater Interfaces 5:7274–7281
Su PG, Shieh HC (2014) Flexible NO2 sensors fabricated by layer-by-layer covalent anchoring and in situ reduction of graphene oxide. Sens Actuators B 190:865–872
Su PG, Lee CT, Chou CY (2009) Flexible NH3 sensors fabricated by in situ self-assembly of polypyrrole. Talanta 80:763–769
Zheng ZQ, Yao JD, Wang B, Yang GW (2015) Light-controlling, flexible and transparent ethanol gas sensor based on ZnO nanoparticles for wearable devices. Sci Rep 5:1–8
Dai ZR, Pan ZW, Wang ZL (2002) Growth and structure evolution of novel tin oxide diskettes. J Am Chem Soc 124:8673–8680
Cheng L, Shao MW, Chen D, Ma DD, Lee ST (2010) SnO2 nanowires with strong yellow emission and their application in photoswitches. Cryst Eng Comm 12(12):1536–1539
Zhang Z, Gao J, Wong LM, Tao JG, Liao L, Zheng Z, Xing GZ, Peng HY, Yu T, Shen ZX, Huan CHA, Wang SJ, Wu T (2009) Morphology-controlled synthesis and a comparative study of the physical properties of SnO2 nanostructures: from ultrathin nanowires to ultrawide nanobelts. Nanotechnology 20(13):135605
Li ZJ, Qin Z, Zhou ZH, Zhang LY, Zhang YF (2009) SnO2 nanowire arrays and electrical properties synthesized by fast heating a mixture of SnO2 and CNTs waste soot. Nanoscale Res Lett 4:1434–1438
Blessi S, Sonia MML, Vijayalakshmi S, Pauline S (2014) Preparation and characterization of SnO2 nanoparticles by hydrothermal method. Int J ChemTech Res 6(3):2153–2155
Wei D, Shen Y, Li M, Liu W, Gao S, Jia L, Han C, Cui B (2013) Synthesis and characterization of single-crystalline SnO2 nanowires. J Nanomater 2013:1–6
El-Maghraby EM, Qurashi A, Yamazaki T (2013) Synthesis of SnO2 nanowires their structural and H2 gas sensing properties. Ceram Int 39(7):8475–8480
Chen YJ, Xiao G, Wang TS, Li QH (2011) Synthesis and enhanced gas sensing properties of crystalline CeO2/TiO2 core/shell nanorods. Sensors Actuators B Chem 156(2):867–874
Comini E, Faglia G, Sberveglier G, Pan Z, Wang ZL (2002) Stable and highly sensitive gas sensors based on semiconducting oxide nanobelts. Appl Phys Lett 81(2):1869–1871
Acknowledgements
This work was supported by Mount Carmel College, Bengaluru, India. The authors would like to thank St.Joseph College, Bengaluru for the XRD and FTIR study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 9.88 mb)
Rights and permissions
About this article
Cite this article
Natarajan, P., Venkatraman, U. & Andrews, N.G. Overhead projector sheets as substrate for deposition of one-dimensional tin dioxide nanostructures for use as a chemoresistive sensor for hydrogen. Microchim Acta 184, 3153–3161 (2017). https://doi.org/10.1007/s00604-017-2329-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2329-6