Abstract
The work describes a photoelectrochemical (PEC) biosensor for the detection of DNA. It employs oriented hierarchical ZnO flower-rod architectures (ZnO FRs) and DNA dendrimers. ZnO FRs act as photoactive material that yields a photocurrent of up to 23 μA. The photogenerated electron transfer is inhibited once the probe DNA and the target DNA oligonucleotides hybridize, and this results in a reduced photocurrent. The use of DNA dendrimers with scores of DNA branches further amplifies the signal of the PEC biosensor. The PEC sensor displays a response that is linear in the DNA concentration range from 10 fM to 0.1 μM with a detection limit of 3.7 fM (at S/N = 3). The sensor was applied to the determination of DNA in human serum samples and was found to work with acceptable accuracy. Due to the use of ZnO FRs and DNA dendrimers, the assay is highly sensitive, rapid, and repeatability.

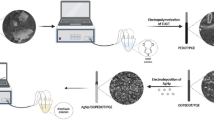
Schematic of an innovative photoelectrochemical (PEC) biosensors based on oriented ZnO flower-rod architectures (ZnO FRs) for DNA detection. Dendrimers were employed for signal amplification to give a low detection limit of 3.7 fM.







Similar content being viewed by others

References
Devadoss A, Sudhagar P, Terashima C, Nakata K, Fujishima A (2015) Photoelectrochemical biosensors: new insights into promising photoelectrodes and signal amplification strategies. J Photochem Photobiol C: Photochem Rev 24:43–63. doi:10.1016/j.jphotochemrev.2015.06.002
Yan K, Wang R, Zhang JD (2014) A photoelectrochemical biosensor for o-aminophenol based on assembling of CdSe and DNA on TiO2 film electrode. Biosens Bioelectron 53:301–304. doi:10.1016/j.bios.2013.09.073
Ge L, Wang PP, Ge SG, Li NQ, Yu JH, Yan M, Huang JD (2013) Photoelectrochemical lab-on-paper device based on an integrated paper supercapacitor and internal light source. Anal Chem 85(8):3961–3970. doi:10.1021/ac4001496
Ibrahim I, Lim H, Abou-Zied O, Huang N, Estrela P, Pandikumar A (2016) Cadmium sulfide nanoparticles decorated with au quantum dots as ultrasensitive photoelectrochemical sensor for selective detection of copper(II) ions. J Phys Chem C 120:22202–22214. doi:10.1021/acs.jpcc.6b06929
Li S, Wang YH, Gao CM, Ge SG, Yu JH, Yan M (2015) “Signal-off” photoelectrochemical DNA sensing strategy based on target dependent DNA probe conformational conversion using CdS quantum dots sensitized TiO2 nanorods array as photoactive material. J Electroanal Chem 759:38–45. doi:10.1016/j.jelechem.2015.06.007
Lu W, Jin Y, Wang G, Chen D, Li JH (2008) Enhanced photoelectrochemical method for linear DNA hybridization detection using au-nanopaticle labeled DNA as probe onto titanium dioxide electrode. Biosens Bioelectron 23(10):1534–1539. doi:10.1016/j.bios.2008.01.011
Zang Y, Lei JP, Ling PH, Ju HX (2015) Catalytic hairpin assembly-programmed porphyrin-DNA complex as photoelectrochemical initiator for DNA biosensing. Anal Chem 87(10):5430–5436. doi:10.1021/acs.analchem.5b00888
Zang Y, Lei JP, Zhang L, Ju HX (2014) In situ generation of electron acceptor for photoelectrochemical biosensing via hemin-mediated catalytic reaction. Anal Chem 86(24):12362–12368. doi:10.1021/ac503741x
Huang JH, Ye L, Gao X, Li H, Xu JB, Li ZG (2015) Molybdenum disulfide-based amplified fluorescence DNA detection using hybridization chain reactions. J Mater Chem B 3(11):2395–2401. doi:10.1039/c4tb01986e
Lou J, Liu SS, Tu WW, Dai ZH (2015) Graphene quantums dots combined with endonuclease cleavage and bidentate chelation for highly sensitive electrochemiluminescent DNA biosensing. Anal Chem 87(2):1145–1151. doi:10.1021/ac5037318
Zhao WW, Xu JJ, Chen HY (2014) Photoelectrochemical DNA biosensors. Chem Rev 114(15):7421–7441. doi:10.1021/cr500100j
Gao ZQ, Tansil NC (2005) An ultrasensitive photoelectrochemical nucleic acid biosensor. Nucleic Acids Res 33(13):e123. doi:10.1093/nar/gni125
Wang M, Yin HS, Shen NN, Xu ZN, Sun B, Ai SY (2014) Signal-on photoelectrochemical biosensor for microRNA detection based on Bi2S3 nanorods and enzymatic amplification. Biosens Bioelectron 53:232–237. doi:10.1016/j.bios.2013.09.069
Bas D, Boyaci IH (2011) Photoelectrochemical competitive DNA hybridization assay using semiconductor quantum dot conjugated oligonucleotides. Anal Bioanal Chem 400(3):703–707. doi:10.1007/s00216-011-4827-4
Shen QM, Han L, Fan GC, Zhang JR, Jiang LP, Zhu JJ (2015) “Signal-on” photoelectrochemical biosensor for sensitive detection of human T-cell lymphotropic virus type II DNA: dual signal amplification strategy integrating enzymatic amplification with terminal deoxynucleotidyl transferase-mediated extension. Anal Chem 87(9):4949–4956. doi:10.1021/acs.analchem.5b00679
Liu F, Zhang Y, Yu JH, Wang SW, Ge SG, Song XR (2014) Application of ZnO/graphene and S6 aptamers for sensitive photoelectrochemical detection of SK-BR-3 breast cancer cells based on a disposable indium tin oxide device. Biosens Bioelectron 51:413–420. doi:10.1016/j.bios.2013.07.066
Foo CY, Lim HN, Pandikumar A, Huang NM, Ng YH (2016) Utilization of reduced graphene oxide/cadmium sulfide-modified carbon cloth for visible-light-prompt photoelectrochemical sensor for copper (II) ions. J Hazard Mater 304:400–408. doi:10.1016/j.jhazmat.2015.11.004
Xia L, Song J, Xu R, Liu DL, Dong B, Xu L, Song HW (2014) Zinc oxide inverse opal electrodes modified by glucose oxidase for electrochemical and photoelectrochemical biosensor. Biosens Bioelectron 59:350–357. doi:10.1016/j.bios.2014.03.038
Wang WJ, Hao Q, Wang W, Bao L, Lei JP, Wang QB, Ju HX (2014) Quantum dot-functionalized porous ZnO nanosheets as a visible light induced photoelectrochemical platform for DNA detection. Nano 6(5):2710–2717. doi:10.1039/c3nr04777f
Tokudome H, Yamada Y, Sonezaki S, Ishikawa H, Bekki M, Kanehira K, Miyauchi M (2005) Photoelectrochemical deoxyribonucleic acid sensing on a nanostructured TiO2 electrode. Appl Phys Lett 87(21):213901–213903. doi:10.1063/1.2135392
Liang MM, Jia SP, Zhu SC, Guo LH (2008) Photoelectrochemical sensor for the rapid detection of in situ DNA damage induced by enzyme-catalyzed fenton reaction. Environ Sci Technol 42(2):635–639. doi:10.1021/es071633h
Zhou XF, Hu ZL, Chen Y, Shang HY (2008) Microscale sphere assembly of ZnO nanotubes. Mater Res Bull 43(10):2790–2798. doi:10.1016/j.materresbull.2007.10.011
Sodzel D, Khranovskyy V, Beni V, Turner A, Viter R, Eriksson M, Holtz P, Janot J-M, Bechelany M, Balme S, Smyntyna V, Koleaneva E, Dubovskaya L, Volotovski I, Ubelis A, Yakimova R (2015) Continuous sensing of hydrogen peroxide and glucose via quenching of the UV and visible luminescence of ZnO nanoparticles. Microchim Acta 182:1819–1826. doi:10.1007/s00604-015-1493-9
Tereshchenko A, Fedorenko V, Smyntyna V, Konup I, Konup A, Eriksson M, Yakimova R, Ramnavicius A, Balme S, Bechelany M (2017) ZnO films formed by atomic layer deposition as an optical biosensor platform for the detection of grapevine virus A-type proteins. Biosens Bioelectron 92:763–769. doi:10.1016/j.bios.2016.09.071
Law M, Greene LE, Johnson JC, Saykally R, Yang PD (2005) Nanowire dye-sensitized solar cells. Nat Mater 4(6):455–459. doi:10.1038/nmat1387
Armelao L, Pascolini M, Biasiolo E, Tondello E, Bottaro G, Carbonare MD, D’Arrigo A, Leon A (2008) Innovative metal oxide-based substrates for DNA microarrays. Inorg Chim Acta 361:3603–3608. doi:10.1016/j.ica.2008.03.121
Lu W, Wang G, Jin Y, Yao X, Hu JQ, Li JH (2006) Label-free photoelectrochemical strategy for hairpin DNA hybridization detection on titanium dioxide electrode. Appl Phys Lett 89(26):263902–263904. doi:10.1063/1.2420786
Xuan F, Hsing IM (2014) Triggering hairpin-free chain-branching growth of fluorescent DNA dendrimers for nonlinear hybridization chain reaction. J Am Chem Soc 136(28):9810–9813. doi:10.1021/ja502904s
Han ZZ, Ren LL, Cui ZH, Chen CQ, Pan HB, Chen JZ (2012) Ag/ZnO flower heterostructures as a visible-light driven photocatalyst via surface plasmon resonance. Appl Catal B Environ 126:298–305. doi:10.1016/j.apcatb.2012.07.002
Kang Z, Yan X, Wang YF, Bai ZM, Liu YC, Zhang Z, Lin P, Zhang XH, Yuan HG, Zhang XJ, Zhang Y (2015) Electronic structure engineering of Cu2O film/ZnO nanorods array all-oxide p-n heterostructure for enhanced photoelectrochemical property and self-powered biosensing application. Sci Report 5:7882–7888. doi:10.1038/srep07882
Takada T, Lin CY, Majima T (2007) Relationship between charge transfer and charge recombination determines photocurrent efficiency through DNA films. Angew Chem Int Ed 46(35):6681–6683. doi:10.1002/anie.200701525
Wu ZF, Li SJ (2012) Infrared spectra characteristics of zinc hydroxide and zinc oxide. Chin J Spectrosc Lab 29:2172–2175. doi:10.3969/j.issn.1004-8138.2012.04.052
Das M, Sumana G, Nagarajan R, Malhotra BD (2010) Application of nanostructured ZnO films for electrochemical DNA biosensor. Thin Solid Films 519(3):1196–1201. doi:10.1016/j.tsf.2010.08.069
Wood BR (2016) The importance of hydration and DNA conformation in interpreting infrared spectra of cells and tissues. Chem Soc Rev 45(7):1980–1998. doi:10.1039/c5cs00511f
Tien CL, Lafortune R, Shareck F, Lacroix M (2007) DNA analysis of a radiotolerant bacterium Pantoea agglomerans by FT-IR spectroscopy. Talanta 71(5):1969–1975. doi:10.1016/j.talanta.2006.09.003
Maruyama T, Katoh S, Nakajima M, Nabetani H, Abbott TP, Shono A, Satoh K (2001) FT-IR analysis of BSA fouled on ultrafiltration and microfiltration membranes. J Membr Sci 192(1):201–207. doi:10.1016/s0376-7388(01)00502-6
Shen GF, Liu TT, Wang Q, Jiang M, Shi JH (2015) Spectroscopic and molecular docking studies of binding interaction of gefitinib, lapatinib and sunitinib with bovine serum albumin (BSA). J Photochem Photobiol B 153(9):380–390. doi:10.1016/j.jphotobiol.2015.10.023
Chen M, Hou CJ, Huo DQ, Bao J, Fa HB, Shen CH (2016) An electrochemical DNA biosensor based on nitrogen-doped graphene/au nanoparticles for human multidrug resistance gene detection. Biosens Bioelectron 85:684–691. doi:10.1016/j.bios.2016.05.051
Li JL, Chen ZP, Xiang Y, Zhou LL, Wang T, Zhang Z, Sun KX, Yin D, Li Y, Xie GM (2016) An electrochemical biosensor for double-stranded Wnt7B genedetection based on enzymatic isothermal amplification. Biosens Bioelectron 86:75–82. doi:10.1016/j.bios.2016.06.031
Gao Y, Li BX (2013) G-quadruplex DNAzyme-based chemiluminescence biosensing strategy for ultrasensitive DNA uetection: combination of exonuclease III-assisted signal amplification and carbon nanotubes-assisted background reducing. Anal Chem 85(23):11494–11500. doi:10.1021/ac402728d
Acknowledgements
The authors gratefully acknowledge the financial support from National Natural Science Foundation of China (51602053 and 21375017), Fujian Natural Science Foundation (2015 J05020), Natural Science Foundation for Distinguished Young Scholars of Fujian Province (2013 J06003), Program for New Century Excellent Talents of Colleges and Universities in Fujian Province (JA13130, JA13088), Youth Scientific Research Program of Fujian Provincial Health and Family Planning Commission (2014-1-39), Nursery Scientific Research Foundation of Fujian Medical University (2014MP008) and Professor Foundation of Fujian Medical University (JS14009).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 9905 kb)
Rights and permissions
About this article
Cite this article
Han, Z., Luo, M., Chen, L. et al. A photoelectrochemical biosensor for determination of DNA based on flower rod-like zinc oxide heterostructures. Microchim Acta 184, 2541–2549 (2017). https://doi.org/10.1007/s00604-017-2257-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2257-5