Abstract
The authors describe a fluorescence based assay for determination of the macrolid antibiotic erythromycin (ERY). It is based in the use of fluorescent gold nanoclusters (AuNCs) coated first with silica (to obtain AuNC@SiO2) and then with a molecularly imprinted polymer (MIP). The MIP was synthesized from 3-aminopropyltriethoxysilane as the functional monomer, ERY as the template, and tetraethoxysilane as the cross-linker via a sol-gel process that leads to surface imprinting. By using this method, the strong fluorescence of AuNCs was maintained in the resultant MIPs. After optimization of the experimental conditions, the signal (measured at excitation/emission wavelengths of 396/585 nm) decreases linearly with increasing concentration of ERY in the 0.1 to 11.9 μM ERY concentration range. The limit of detection is 12 nM, and the imprinting factor is 4.0. The method was successfuly applied to the determination of ERY in spiked human urine and saliva.

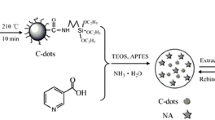
Schematic of the preparation of a core-shell sensor material with the silica coated by fluorescent gold nanoclusters as core and a molecularly imprinted polymer. It can be used to selectively detect residual erythromycin in biological samples.







Similar content being viewed by others
References
Zuckerman JK (2016) Macrolides and ketolides: azithromycin, clarithromycin, telithromycin. Infect Dis Clin N Am 18:621–649
Pal S (2006) A journey across the sequential development of macrolides and ketolides related to erythromycin. ChemInform 62:3171–3200
Kees F, Spangler S, Wellenhofer M (1998) Determination of macrolides in biological matrices by high performance liquid chromatography with electrochemical detection. J Chromatogr 812:287–293
Lian W, Liu S, Yu J, Xing X, Li J, Cui M, Huang J (2012) Electrochemical sensor based on gold nanoparticles fabricated molecularly imprinted polymer film at chitosan-platinum nanoparticles/graphene-gold nanoparticles double nanocomposites modified electrode for detection of erythromycin. Biosens Bioelectron 38:163–169
Tsuji K, Kane MP (1982) Improved high-pressure liquid chromatographic method for the analysis of erythromycin in solid dosage forms. J Pharm Sci 71:1160–1164
Liawruangrath B, Liawruangrath S (2001) High performance thin layer chromatographic determination of erythromycin in pharmaceutical preparations. Chromatographia 54:405–408
Wardrop J, Ficker D, Franklin S, Gorski RJ (2000) Determination of erythromycin and related substances in enteric-coated tablet formulations by reversed-phase liquid chromatography. J Pharm Sci 89:1097–1105
Gunes N, Cibik R, Gunes ME, Aydin L (2008) Erythromycin residue in honey from the southern Marmara region of Turkey food additives and contaminants part. A Chemistry Analysis Control Exposure and Risk Assessment 25:1313–1317
Hassib ST, Farag AE, Elkady EF (2011) Liquid chromatographic and spectrophotometric methods for the determination of erythromycin stearate and trimethoprim in tablets. Bulletin of Faculty of Pharmacy Cairo University 49:81–89
Cachet T, Delrue M, Paesen J, Busson R, Roets E (1992) Hoogmartens, analysis of erythromycin estolate by liquid chromatography. J Pharm Biomed Anal 10:851–860
Feng YC, Hu CQ (2006) Construction of universal quantitative models for determination of roxithromycin and erythromycin ethylsuccinate in tablets from different manufacturers using near infrared reflectance spectroscopy. J Pharm Biomed Anal 41:373–384
Tepe J, John St C (1955) Determination of erythromycin by ultraviolet spectrophotometry. Anal Chem 27:744–746
Li JH, Zhang Z, Xu SF, Chen LX, Zhou N, Xiong H, Peng HL (2011) Label-free colorimetric detection of trace cholesterol based on molecularly imprinted photonic hydrogels. J Mater Chem 21:19267–19274
Wulff G (2013) Fourty years of molecular imprinting in synthetic polymers: origin, features and perspectives. Microchim Acta 180:1359–1370
Wu YL, Lu J, Meng MJ, Dai JD, Lin XY, Gao J, Li CX, Yan YS (2017) Bioinspired synthesis of pDA/SiO2-based porous ciprofloxacin-imprinted nanocomposite membrane by a polydopamine-assisted organic-inorganic method. Chem Eng J 309:263–267
Wu YL, Zhao J, Wang C, Lu J, Meng MJ, Dai XH, Yan YS, Li CX (2016) A novel approach toward fabrication of porous molecularly imprinted nanocomposites with bioinspired multilevel internal domains: application to selective adsorption and separation membrane. Chem Eng J 306:492–503
Xu XF, Li JH, Chen LX (2011) Molecularly imprinted core-shell nanoparticles for determination of trace atrazine by reversible addition-fragmentation chain transfer surface imprinting. J Mater Chem 21:4346–4351
Song XL, Li JH, Xu SF, Ying R, Ma J, Liao C, Liu D, Yu J, Chen LX (2012) Determination of 16 polycyclic aromatic hydrocarbons in seawater using molecularly imprinted solid-phase extraction coupled with gas chromatography-mass spectrometry. Talanta 99:75–82
Chen LX, Xu SF, Li JH (2011) Recent advances in molecular imprinting technology: current status, challenges and highlighted applications. Chem Soc Rev 40:2922–2942
Wei X, Hao T, Xu Y, Lu K, Li H, Yan Y, Zhou Z (2015) Facile polymerizable surfactant inspired synthesis of fluorescent molecularly imprinted composite sensor via aqueous CdTe quantum dots for highly selective detection of λ-cyhalothrin. Sensors Actuators B Chem 224:315–324
Li Q, Su HJ, Tan TW (2008) Synthesis of ion-imprinted chitosan-TiO2 adsorbent and its multi-functional performances. Biochem Eng J 38:212–218
Xie CG, Zhang ZP, Wang DP, Guan GJ, Gao DM, Liu JH (2006) Surface molecular self-assembly strategy for TNT imprinting of polymer nanowire/nanotube arrays. Anal Chem 78:8339–8346
Dai JD, Wei X, Cao ZJ, Zhou ZP, Yu P, Pan JM, Zou TB, Li CX, Yan YS (2014) Highly-controllable imprinted polymer nanoshell at the surface of magnetic halloysite nanotubes for selective recognition and rapid adsorption of tetracycline. RSC Adv 4:7967–7978
Zheng J, Nicovich PR, Dickson RM (2007) Highly fluorescent noble-metal quantum dots. Annu Rev Phys Chem 58:409–431
Wu YL, Wang C, Zhao J, Cui JY, Yan YS, Li CX (2016) Accelerating the design of gold/polymers/silica-based imprinted nanocomposite for light-triggered recognition and separation of biomolecules. Chem Eng J 307:621–630
Li JJ, Zhu JJ, Xu K (2014) Fluorescent metal nanoclusters: from synthesis to applications. TrAC Trends Anal Chem 58:90–98
Zhang W, He XW, Chen Y, Li WY, Zhang YK (2012) Molecularly imprinted polymer anchored on the surface of denatured bovine serum albumin modified CdTe quantum dots as fluorescent artificial receptor for recognition of target protein. Biosens Bioelectron 31:84–89
Wei X, Zhou ZP, Hao TF, Xu YQ, Li HJ, Lu K, Dai JD, Zheng XD, Gao L, Wang JX, Yan YS, Zhu YZ (2015) Specific recognition and fluorescent determination of aspirin by using core-shell CdTe quantum dot-imprinted polymers. Microchim Acta 182:1527–1534
Li HB, Li YL, Cheng J (2010) Molecularly imprinted silica nanospheres embedded CdSe quantum dots for highly selective and sensitive optosensing of pyrethroids. Chem Mater 22:2451–2457
Chen TT, Hu YH, Cen Y, Chu X, Lu Y (2013) A dual–emission fluorescent nanocomplex of gold-cluster-decorated silica particles for live cell imaging of highly reactive oxygen species. J Am Chem Soc 135:11595–11602
Hu LZ, Han S, Parveen S, Yuan YL, Zhang L, Xu GB (2012) Highly sensitive fluorescent detection of trypsin based on BSA-stabilized gold nanoclusters. Biosens Bioelectron 32:297–299
Liu HY, Zhang X, Wu XM, Jiang LP, Burda C, Zhu JJ (2011) Rapid sonochemical synthesis of highly luminescent non-toxic AuNCs and au@AgNCs and cu(II) sensing. Chem Inform 42(27):4237–4239
Lin H, Li LJ, Lei CY, Xu XH, Nie Z, Guo ML, Huang Y, Yao SZ (2013) Immune-independent and label-free fluorescent assay for cystatin Cdetection based on protein-stabilized au nanoclusters. Biosens Bioelectron 41:256–261
Wu XQ, Zhang Z, Li JH, You HY, Li YB, Chen LX (2015) Molecularly imprinted polymers-coated gold nanoclusters for fluorescent detection of bisphenol a. Sensors Actuators B Chem 211:507–514
Stober W, Fink A (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci 26:62–69
Luo ZT, Yuan X, Yu Y, Zhang QB, Long DT, Lee JY, Xie JP (2012) From aggregation-induced emission of au(I)-thiolate complexes to ultrabright au(0)@au(I)-thiolate core-shell nanoclusters. J Am Chem Soc 134:16662–16670
Zhang K, Zhou HB, Mei QS, Wang SH, Guan GJ, Liu RY, Zhang J, Zhang ZP (2011) Instant visual detection of trinitrotoluene particulates on various surfaces by ratiometric fluorescence of dual-emission quantum dots hybrid. J Am Chem Soc 133:8424–8427
Chen LY, Chen CL, Li RN, Li Y, Liu SQ (2009) CdTe quantum dot functionalized silica nanosphere labels for ultrasensitive detection of biomarker. Chem Commun 19:2670–2672
Lin Z, Xia ZW, Zheng JN, Zheng D, Zhang L, Yang HH, Chen GN (2012) Synthesis of uniformly sized molecularly imprinted polymer-coated silica nanoparticles for selective recognition and enrichment of lysozyme. J Mater Chem 22:17914–17922
Xiao ZH, Peng WG, Yang JQ, Zhang B, Li J, Luo J, Wu KB (2010) Enhanced electrochemical detection of erythromycin based on acetylene black nanoparticles. Colloids Surf B: Biointerfaces 81:27–31
Ganjali MR, Pirzadeh-Naeeni S, Faridbod F, Attar H, Hosseini M (2011) Nano-composite carbon paste electrode and PVC membrane sensor for potentiometric determination of erythromycin. Int J Electrochem Sci 6:1968–1980
Jacobs M, Nagaraj VJ, Mertz T, Selvam AP, Ngo T, Prasad S (2013) An electrochemical sensor for the detection of antibiotic contaminants in water. Anal Methods 5:4325–4329
Song B, Zhou Y, Jin H, Jing T, Zhou T, Hao Q, Zhou Y, Mei S, Lee Y (2014) Selective and sensitive determination of erythromycin in honey and dairy products by molecularly imprinted polymers based. Microchem J 116:183–190
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. U1507118, U1407123, 21406085) and Natural Science Foundation of Jiangsu Province (Nos. BK20151350, BK20140580, BK20161367), China Postdoctoral Science Foundation Funded Project (Nos. 2014 M561588, 1501067C).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 189 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Zhou, Z., Zheng, J. et al. SiO2-MIP core-shell nanoparticles containing gold nanoclusters for sensitive fluorescence detection of the antibiotic erythromycin. Microchim Acta 184, 2241–2248 (2017). https://doi.org/10.1007/s00604-017-2216-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2216-1