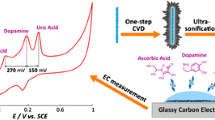
Abstract
The authors describe a voltammetric sensor for simultaneous determination of dopamine (DA), uric acid (UA), L-tyrosine (Tyr), and the diuretic drug hydrochlorothiazide (HCTZ). The assay is based on the use of graphene nanowalls deposited on a tantalum substrate. The nanowalls are characterized by scanning electron microscopy, transmission electron microscopy, X-ray diffraction, electrochemical impedance spectroscopy, and cyclic voltammetry. The nanowalls are vertically grown on the substrate by direct-current arc plasma jet chemical vapor deposition. The modified electrode is shown to enable simultaneous differential pulse voltammetric determination of DA, UA, Tyr, and HCTZ. The graphene nanowalls display a large specific surface, high conductivity, and a large number of catalytically active sites for oxidation of analytes. Simultaneous detection is performed best at a pH value of 7.0 and at peak potentials of 0.124 V (vs. SCE) for DA, 0.256 V for UA, 0.536 V for Tyr and 0.708 V for HCTZ. The respective detection limits are 0.04 μM, 0.1 μM, 0.6 μM and 0.4 μM. The results show that this graphene wall modified electrode is a promising tool for the design of sensitive, selective, and stable sensors.

The graphene-based differential pulse voltammetric sensor for simultaneous determination of dopamine, uric acid, L-tyrosine, and hydrochlorothiazide exhibits high selectivity, sensitivity, and stability.







Similar content being viewed by others
References
Sanghavi BJ, Wolfbeis OS, Hirsch T, Swami NS (2015) Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. Microchim Acta 182:1–41
Messerli FH, Makani H, Benjo A, Romero J, Alviar C, Bangalore S (2011) Antihypertensive efficacy of hydrochlorothiazide as evaluated by ambulatory blood pressure monitoring: a meta-analysis of randomized trials. J Am Coll Cardiol 57:590–600
Vojta J, Jedlička A, Coufal P, Janečková L (2015) A new, rapid, stability-indicating UPLC method for separation and determination of impurities in amlodipine besylate, valsartan and hydrochlorothiazide in their combined tablet dosage form. J Pharm Biomed Anal 109:36–44
Schroll H, Beste C, Hamker FH (2015) Combined lesions of direct and indirect basal ganglia pathways but not changes in dopamine levels explain learning deficits in patients with Huntington's disease. Eur J Neurosci 41:1227–1244
van Wouwe NC, Kanoff KE, Claassen DO, Spears CA, Neimat J, van den Wildenberg WPM, Wylie SA (2016) Dissociable effects of dopamine on the initial capture and the reactive inhibition of impulsive actions in Parkinson's disease. J Cogn Neurosci 28:710–723
Cagli K, Turak O, Canpolat U, Ozcan F, Tok D, Mendi MA, Oksuz F, Siriopol D, Veisa G, Covic A, Kanbay M (2015) Association of Serum Uric Acid Level with Blood Pressure Variability in newly diagnosed essential hypertension. J Clin Hypertens 17:929–935
Li Y, Ran G, Yi WJ, Luo HQ, Li NB (2012) A glassy carbon electrode modified with graphene and poly(acridine red) for sensing uric acid. Microchim Acta 178:115–121
Yokuş ÖA, Kardaş F, Akyıldırım O, Eren T, Atar N, Yola ML (2016) Sensitive voltammetric sensor based on polyoxometalate/reduced graphene oxide nanomaterial: application to the simultaneous determination of l-tyrosine and l-tryptophan. Sensors Actuators B Chem 233:47–54
Joshi A, Schuhmann WC, Nagaiah T (2016) Mesoporous nitrogen containing carbon materials for the simultaneous detection of ascorbic acid, dopamine and uric acid. Sensors Actuators B Chem 230:544–555
Mansano GR, Pires Eisele AP, Sartori ER (2015) Electrochemical evaluation of a boron-doped diamond electrode for simultaneous determination of an antihypertensive ternary mixture of amlodipine, hydrochlorothiazide and valsartan in pharmaceuticals. Anal Methods 7:1053–1060
Taei M, Hasanpour F, Salavati H, Banitaba SH, Kazemi F (2016) Simultaneous determination of cysteine, uric acid and tyrosine using Au-nanoparticles/poly(E)-4-(p-tolyldiazenyl)benzene-1,2,3-triol film modified glassy carbon electrode. Mater Sci Eng C 59:120–128
Palanisamy S, Ku SH, Chen SM (2013) Dopamine sensor based on a glassy carbon electrode modified with a reduced graphene oxide and palladium nanoparticles composite. Microchim Acta 180:1037–1042
Zhu XH, Jiao QF, Zhang CY, Zuo XX, Xiao X, Liang Y, Nan JM (2013) Amperometric nonenzymatic determination of glucose based on a glassy carbon electrode modified with nickel(II) oxides and graphene. Microchim Acta 180:477–483
Yang X, Feng B, He XL, Li FP, Ding YL, Fei JJ (2013) Carbon nanomaterial based electrochemical sensors for biogenic amines. Microchim Acta 180:935–956
Kannan PK, Moshkalev SA, Rout CS (2016) Highly sensitive and selective electrochemical dopamine sensing properties of multilayer graphene nanobelts. Nanotechnology 27:075504
Park S, Hwang S, Takenaka S, Kim K (2015) Electrochemical sensing performances for uric acid detection on various amine Adlayers used in immobilizing reduced graphene oxide. Electroanalysis 27:1159–1165
Yola ML, Eren T, Atar N (2015) A sensitive molecular imprinted electrochemical sensor based on gold nanoparticles decorated graphene oxide: application to selective determination of tyrosine in milk. Sensors Actuators B Chem 210:149–157
Er E, Celikkan H, Aksu ML, Erk N (2015) A novel and highly sensitive electrochemical sensor based on a high-quality modified graphene electrode for the determination of hydrochlorothiazide in pharmaceutical formulations and human plasma. Anal Methods 7:9254–9260
Abdelwahab AA, Shim YB (2015) Simultaneous determination of ascorbic acid, dopamine, uric acid and folic acid based on activated graphene/MWCNT nanocomposite loaded Au nanoclusters. Sensors Actuators B Chem 221:659–665
Chae SJ, Guenes F, Kim KK, Kim ES, Han GH, Kim SM, Shin H-J, Yoon S-M, Choi J-Y, Park MH, Yang CW, Pribat D, Lee YH (2009) Synthesis of large-area graphene layers on poly-nickel substrate by chemical vapor deposition: wrinkle formation. Adv Mater 21:2328–2333
Michon A, Tiberj A, Vezian S, Roudon E, Lefebvre D, Portail M, Zielinski M, Chassagne T, Camassel J, Cordier Y (2014) Graphene growth on AlN templates on silicon using propane-hydrogen chemical vapor deposition. Appl Phys Lett 104
Syed Muhammad H, Chong SK, Huang NM, Abdul Rahman S (2015) Fabrication of high-quality graphene by hot-filament thermal chemical vapor deposition. Carbon 86:1–11
Randles JEB (1948) A cathode ray polarograph. Part II.-the current-voltage curves. Trans Faraday Soc 44:327–338
Nicholson RS, Shain I (1964) Theory of stationary electrode polarography. Single scan and cyclic methods applied to reversible, irreversible, and kinetic systems. Anal Chem 36:706–723
Deng K, Li X, Huang H (2016) A glassy carbon electrode modified with a nickel(II) norcorrole complex and carbon nanotubes for simultaneous or individual determination of ascorbic acid, dopamine, and uric acid. Microchim Acta 183:2139–2145
Li Y, Lin H, Peng H, Qi R, Luo C (2016) A glassy carbon electrode modified with MoS2 nanosheets and poly(3,4-ethylenedioxythiophene) for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. Microchim Acta 183:2517–2523
Liu R, Zeng X, Liu J, Luo J, Zheng Y, Liu X (2016) A glassy carbon electrode modified with an amphiphilic, electroactive and photosensitive polymer and with multi-walled carbon nanotubes for simultaneous determination of dopamine and paracetamol. Microchim Acta 183:1543–1551
Mei L-P, Feng J-J, Wu L, Chen J-R, Shen L, Xie Y, Wang A-J (2016) A glassy carbon electrode modified with porous Cu2O nanospheres on reduced graphene oxide support for simultaneous sensing of uric acid and dopamine with high selectivity over ascorbic acid. Microchim Acta 183:2039–2046
Ming L, Peng T, Tu Y (2016) Multiple enhancement of luminol electrochemiluminescence using electrodes functionalized with titania nanotubes and platinum black: ultrasensitive determination of hydrogen peroxide, resveratrol, and dopamine. Microchim Acta 183:305–310
Tsierkezos NG, Ritter U, Thaha YN, Downing C, Szroeder P, Scharff P (2016) Multi-walled carbon nanotubes doped with boron as an electrode material for electrochemical studies on dopamine, uric acid, and ascorbic acid. Microchim Acta 183:35–47
Wu F, Huang T, Hu Y, Yang X, Ouyang Y, Xie Q (2016) Differential pulse voltammetric simultaneous determination of ascorbic acid, dopamine and uric acid on a glassy carbon electrode modified with electroreduced graphene oxide and imidazolium groups. Microchim Acta 183:2539–2546
Xing LW, Ma ZF (2016) A glassy carbon electrode modified with a nanocomposite consisting of MoS2 and reduced graphene oxide for electrochemical simultaneous determination of ascorbic acid, dopamine, and uric acid. Microchim Acta 183:257–263
Yan SL, Li X, Xiong Y, Wang MM, Yang LL, Liu X, Li XY, Alshahrani LAM, Liu P, Zhang CC (2016) Simultaneous determination of ascorbic acid, dopamine and uric acid using a glassy carbon electrode modified with the nickel(II)-bis(1,10-phenanthroline) complex and single-walled carbon nanotubes. Microchim Acta 183:1401–1408
Zhao D, Fan D, Wang J, Xu C (2015) Hierarchical nanoporous platinum-copper alloy for simultaneous electrochemical determination of ascorbic acid, dopamine, and uric acid. Microchim Acta 182:1345–1352
Gimenes DT, Marra MC, de Freitas JM, Abarza Muñoz RA, Richter EM (2015) Simultaneous determination of captopril and hydrochlorothiazide on boron-doped diamond electrode by batch injection analysis with multiple pulse amperometric detection. Sensors Actuators B Chem 212:411–418
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 61301045, 61401306 and 61504096), the Natural Science Foundation of Tianjin City (Nos. 15JCYBJC24000, 16JCYBJC16300, and 16JCTPJC50800), and the Youth Top-notch Talents Program of Tianjin.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 4236 kb)
Rights and permissions
About this article
Cite this article
Tian, F., Li, H., Li, M. et al. A tantalum electrode coated with graphene nanowalls for simultaneous voltammetric determination of dopamine, uric acid, L-tyrosine, and hydrochlorothiazide. Microchim Acta 184, 1611–1619 (2017). https://doi.org/10.1007/s00604-017-2154-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2154-y