Abstract
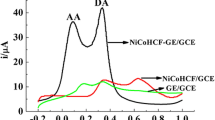
A glassy carbon electrode was modified with a composite consisting of ferrocene imidazolium salts and hydroxy-functionalized graphene in a Nafion matrix. The electrode is shown to facilitate the reduction of oxygen to form the superoxide anion radical. Atomic force microscopy and electrochemical impedance spectroscopy were used to characterize the electrode. The in-situ production of superoxide anion was then studied with an analogue of methyl-Cypridina luciferin (also referred to as Vargulin). As a result, an electrochemiluminescence (ECL) based assay was developed for the improved determination of the activity of the enzyme superoxide dismutase (SOD). Under optimized conditions, the ECL based calibration plot is linear in the 0.5 to 6.5 U·mL−1 SOD activity range, with a 0.2 U·mL−1 detection limit (at a signal-to-noise ratio of 3).

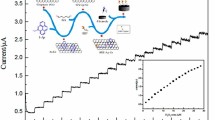
Schematic of the detection scheme. The surface of a glassy carbon electrode is modified with a modified ferrocenyl-methyl-imidazolium ionic liquid and hydroxy-functionalized graphene. The release of superoxide radical is detected via the electrochemiluminescence of MCLA (a methyl cypridina luciferin analogue)








Similar content being viewed by others

References
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669. doi:10.1126/science.1102896
Ratinac KR, Yang WR, Gooding JJ, Thordarson P, Braet F (2011) Graphene and related materials in electrochemical sensing. Electroanal 23:803–826. doi:10.1002/elan.201000545
Pei S, Zhao J, Du J, Ren W, Cheng HM (2010) Direct reduction of graphene oxide films into highly conductive and flexible graphene films by hydrohalic acids. Carbon 48:4466–4474. doi:10.1016/j.carbon.2010.08.006
Yusoff N, Pandikumar A, Marlinda AR, Huang NM, Lim HN (2015) Facile synthesis of nanosized graphene/Nafion hybrid materials and their application in electrochemical sensing of nitric oxide. Anal Methods 7:3537–3544. doi:10.1039/C5AY00604J
Marlinda AR, Pandikumar A, Yusoff N, Huang NM, Lim HN (2015) Electrochemical sensing of nitrite using a glassy carbon electrode modified with reduced functionalized graphene oxide decorated with flower-like zinc oxide. Microchim Acta 182:1113. doi:10.1007/s00604-014-1436-x
Zeng JX, Wei WZ, Wu L, Liu XY, Liu K, Li Y (2006) Fabrication of poly(toluidine blue O)/carbon nanotube composite nanowires and its stable low-potential detection of NADH. J Electroanal Chem 595:152–160. doi:10.1016/j.jelechem.2006.07.014
Sun W, Wang Y, Zhang Y, Ju X, Li G, Sun Z (2012) Poly(methylene blue) functionalized graphene modified carbon ionic liquid electrode for the electrochemical detection of dopamine. Anal Chim Acta 751:59–65. doi:10.1016/j.aca.2012.09.006
Dong Y, Wu H, Shang P, Chi Y (2015) Immobilizing water-soluble graphene quantum dots with gold nanoparticles for a low potential electrochemiluminescence immunosensor. Nanoscale 7:16366–16371. doi:10.1039/C5NR04328J
Cristarella TC, Chinderle AJ, Hui J, Rodríguez-Lopez J (2015) Single-layer graphene as a stable and transparent electrode for nonaqueous radical annihilation electrogenerated chemiluminescence. Langmuir 31(13):3999–4007. doi:10.1021/la5050317
Hosseini M, Mirzanasiri N, Rezapour M, Sheikhha MH, Faridbod F, Norouzi P, Ganjali MR (2015) Sensitive determination of carbidopa through the electrochemiluminescence of luminol at graphene-modified electrodes. Luminescence 30(4):376–381. doi:10.1002/bio.2742
Liu YM, Shi GF, Zhang JJ et al (2014) A novel label-free electrochemiluminescence aptasensor based on layered flowerlike molybdenum sulfide-graphene nanocomposites as matrix. Colloids Surf B Biointerfaces 122:287–293. doi:10.1016/j.colsurfb.2014.07.011
Fattman CL, Enghild JJ, Crapo JD, Schaefer LM, Valnickova Z, Oury TD (2000) Purification and characterization of extracellular superoxide dismutase in mouse lung. Biochem Biophys Res Commun 275:542–548. doi:10.1006/bbrc.2000.3327
Haddad NIA, Yuan Q (2005) Purification and some properties of Cu, Zn superoxide dismutase from radix lethospermi seed, kind of chinese traditional medicine. J Chromatogr B 818:123–131. doi:10.1016/j.jchromb.2004.12.010
Oztürk-Urek R, Tarhan L (2001) Purification and characterization of superoxide dismutase from chicken liver. Comp Biochem Physiol B Biochem Mol Biol 128:205–212. doi:10.1016/S1096-4959(00)00300-6
Chen Z, Wang J, Lin Z, Chen G (2006) Electrogenerated chemiluminescent behavior of MCLA at an indium-tin-oxide electrode. Electrochim Acta 51:5864–5869. doi:10.1016/j.electacta.2006.03.022
Hummers J, Offeman WS (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339. doi:10.1021/ja01539a017
Moon IK, Lee J, Ruoff RS, Lee H (2010) Reduced graphene oxide by chemical graphitization. Nat Commun 1:73. doi:10.1038/ncomms1067
Biniak S, Szymanski G, Siedlewski J, Swiatkowski A (1997) The characterization of activated carbons with oxygen and nitrogen surface groups. Carbon 35:1799–1810. doi:10.1016/S0008-6223(97)00096-1
Si Y, Samulski T (2008) Synthesis of water soluble graphene. Nano Lett 8:1679–1682. doi:10.1021/nl080604h
Zeng F, Sun Z, Sang X, Diamond D, Lau KT, Liu X, Su DS (2011) In situ one-step electrochemical preparation of graphene oxide nanosheet-modified electrodes for biosensors. ChemSusChem 4:1587–1591. doi:10.1002/cssc.201100319
Ghaderi N, Peressi M (2010) First-principle study of hydroxyl functional groups on pristine, defected graphene, and graphene epoxide. J Phys Chem C 114:21625–21630. doi:10.1021/jp108688m
Xu Y, Bai H, Lu G, Li C, Shi G (2008) Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J Am Chem Soc 130:5856–5857. doi:10.1021/ja800745y
Yang H, Jiang J, Zhou W, Lai L, Xi L, Lam Y, Shen Z, Khezri B, Yu T (2011) Influences of graphene oxide support on the electrochemical performances of graphene oxide-MnO2 nanocomposites. Nanoscale Res Lett 6:531. doi:10.1186/1556-276X-6-531
Li D, Muller MB, Gilje S, Kaner RB, Wallace GG (2008) Processable aqueous dispersions of grapheme nanosheets. Nat Nanotechnol 3:101–105. doi:10.1038/nnano.2007.451
Abuelteen KH, Abdeljalil RJ, Hamad MA, Ghaleb M, Khan KM, Voelter W (2008) Fungicidal effects of some derivatives of 2-ferrocenyl-benzimidazoles: a possible template for antifungal drug design. J Med Sci 8:673. doi:10.3923/jms.2008.673.681
Snegur LV, Nekrasov YS, Sergeeva NS, Zhilina ZV, Gurnenyuk VV, Starikova ZA, Simenel AA, Morozova NB, Sviridova IK, Babin VN (2008) Ferrocenylalkyl azoles: bioactivity, synthesis, structure. Appl Organomet Chem 22:139–147. doi:10.1002/aoc.1362
Akpinar H, Balan A, Baran D, Unver EK, Toppare L (2010) Donor–acceptor–donor type conjugated polymers for electrochromic applications: benzimidazole as the acceptor unit. Polymer 51:6123–6131. doi:10.1016/j.polymer.2010.10.045
Dashtestani F, Ghourchian H, Eskandari K, Rafiee-Pour HA (2014) A superoxide dismutase mimic nanocomposite for amperometric sensing of superoxide anions. Microchim Acta 182:1045–1053. doi:10.1007/s00604-014-1424-1
Lvovich V, Scheeline A (1997) Amperometric sensors for simultaneous superoxide and hydrogen peroxide detection. Anal Chem 69:454–462. doi:10.1021/ac9606261
Mesaros S, Vankova Z, Grunfeld S, Mesarosova A, Malinski T (1998) Preparation and optimization of superoxide microbiosensor. Anal Chim Acta 358:27–33. doi:10.1016/S0003-2670(97)00589-8
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. doi:10.1016/0003-2697(71)90370-8
Corbisier P, Houbion A, Remacle J (1987) A new technique for highly sensitive detection of superoxide dismutase activity by chemiluminescence. Anal Biochem 164:240–247. doi:10.1016/0003-2697(87)90392-7
Lissi E, Pascual C, Castillo MD (1994) On the use of the quenching of luminol luminescence to evaluate sod activity. Free Radic Biol Med 16:833–837. doi:10.1016/0891-5849(94)90200-3
Zhidkova TV, Proskurnina EV, Parfenov EA, Vladimirov YA (2011) Determination of superoxide dismutase and SOD-mimetic activities by a chemical system:Co2/H2O2/lucigenin. Anal Bioanal Chem 401:381. doi:10.1007/s00216-011-5070-8
Rose AL, Moffett JW, Waite TD (2008) Determination of superoxide in seawater using 2-methyl-6-(4-methoxyphenyl)-3,7-dihydroimidazo[1,2-a]pyrazin-3(7H)-one chemiluminescence. Anal Chem 80:1215–1227. doi:10.1021/ac7018975
Acknowledgments
This project was financially supported by the Scientific and Technological Foundation of Fujian Province of China (2011 J01043), Education Ministry of Fujian Province of China (JA15057) and Fuzhou University (2014-XQ-8).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 443 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Jiang, Z., Xie, L. et al. Determination of the activity of superoxide dismutase using a glassy carbon electrode modified with ferrocene imidazolium salts and hydroxy-functionalized graphene. Microchim Acta 184, 289–296 (2017). https://doi.org/10.1007/s00604-016-2018-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-2018-x