Abstract
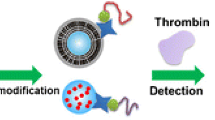
We report on the preparation of fluorescent silica nanoparticles (SiNPs) modified with chitosan and lucigenin by using a reverse microemulsion method. The introduction of chitosan to the lucigenin doped SiNPs is shown to improve the fluorescence quantum yield. The modified SiNPs were used as fluorescent markers in an aptamer-based method for selective determination of thrombin. In this protocol, thrombin was sandwiched between streptavidin-coated magnetic beads and the fluorescent SiNPs modified with a thrombin-binding aptamer. The method was successfully applied to the determination of thrombin in human serum and showed a detection limit as low as 0.02 nM. In our perception, the protocol presented here is promising in that such SiNPs may be applied to the sensitive fluorescent detection of other analytes by changing the corresponding aptamer.

The introduction of chitosan to the lucigenin doped SiNPs is shown to improve the fluorescence quantum yield. The modified SiNPs were used as fluorescent markers in an aptamer-based method for selective determination of thrombin. The effect of chitosan concentration on fluorescence intensity of lucigenin/SiO2 nanoparticles (the volume of chitosan solution is 100 μL)







Similar content being viewed by others
References
Gao J, Chen K, Luong R, Bouley D, Mao H, Qiao T, Gambhir S, Cheng Z (2012) A novel clinically translatable fluorescent nanoparticle for targeted molecular imaging of tumors in living subjects. Nano Lett 12:281–286
Tang F, He F, Cheng H, Li L (2010) Self-assembly of conjugated polymer-Ag@SiO2 hybrid fluorescent nanoparticles for application to cellular imaging. Langmuir 26:11774–11778
Linares E, Pannuti C, Kubota L, Thalhammer S (2013) Immunospot assay based on fluorescent nanoparticles for Dengue fever detection. Biosens Bioelectron 41:180–185
Huang X, Aguilar Z, Li H, Lai W, Wei H, Xu H, Xiong Y (2013) Fluorescent Ru(phen)3 2+- doped silica nanoparticles-based ICTS sensor for quantitative detection of enrofloxacin residues in chicken meat. Anal Chem 85:5120–5128
Bagwe R, Yang C, Hilliard L, Tan W (2004) Optimization of dye-doped silica nanoparticles prepared using a reverse microemulsion method. Langmuir 20:8336–8342
Ow H, Larson D, Srivastava M, Baird WW, Wiesner U (2005) Bright and stable core-shell fluorescent silica nanoparticles. Nano Lett 5:113–117
Zhao X, Bagwe R, Tan W (2004) Development of organic-dye-doped silica nanoparticles in a reverse microemulsion. Adv Mater 16:173–176
Wang L, Wang K, Santra S, Zhao X, Hilliard L, Smith J, Wu J, Tan W (2006) Watching silica nanoparticles glow in the biological world. Anal Chem 78:646–654
Tan W, Wang K, He X, Zhao XJ, Drake T, Wang L, Bagwe RP (2004) Bionanotechnology based on silica nanoparticles. Med Res Rev 24:621–638
Yao G, Wang L, Wu Y, Smith J, Xu J, Zhao W, Lee E, Tan W (2006) FloDots: luminescent nanoparticles. Anal Bioanal Chem 385:518–524
Bakalova R, Zhelev Z, Aoki I, Masamoto K, Mileva M, Obata T, Higuchi M, Gadjeva V, Kanno I (2008) Multimodal silica-shelled quantum dots: direct intracellular delivery, photosensitization, toxic, and microcirculation effects. Bioconjugate Chem 19:1135–1142
Zou H, Wu SS, Shen J (2008) Polymer/silica nanocomposites: preparation, characterization, properties, and applications. Chem Rev 108:3893–3957
Piao Y, Burns A, Kim J, Wiesner U, Hyeon T (2008) Designed fabrication of silica-based nanostructured particle systems for nanomedicine applications. Adv Funct Mater 18:1–14
Santra S, Zhang P, Wang K, Tapec R, Tan W (2001) Conjugation of biomolecules with luminophore-doped silica nanoparticles for photostable biomarkers. Anal Chem 73:4988–4993
Ow H, Larson D, Srivastava M, Baird B, Webb W, Wiesner U (2005) Bright and stable core-shell fluorescent silica nanoparticles. Nano Lett 5:113–117
Lian W, Litherland S, Badrane H, Tan W, Wu D, Baker H, Gulig P, Lim JS (2004) Ultrasensitive detection of biomolecules with fluorescent dye-doped nanoparticles. Anal Biochem 334:135–144
Rossi L, Shi L, Quina F, Rosenzweig Z (2005) Stoeber synthesis of monodispersed luminescent silica nanoparticles for bioanalytical assays. Langmuir 21:4277–4280
Bonacchi S, Genovese D, Juris R, Montalti M, Prodi L, Rampazzo E, Zaccheroni N (2011) Luminescent silica nanoparticles: extending the frontiers of brightness. Angew Chem Int Ed 50:4056–4066
Larson S, Ow H, Vishwasrao H, Heikal A, Wiesner U, Webb W (2008) Silica nanoparticle architecture determines radiative properties of encapsulated fluorophores. Chem Mater 20:2677–2684
Aslan K, Wu M, Lakowicz J, Geddes C (2007) Fluorescent core-shell Ag@SiO2 nanocomposites for metal-enhanced fluorescence and single nanoparticle sensing platforms. J Am Chem Soc 129:1524–1525
Martini M, Perriat P, Montagna M, Pansu R, Julien C, Tillement O, Roux S (2009) How gold particles suppress concentration quenching of fluorophores encapsulated in silica beads. J Phys Chem C 113:17669–17677
Tian R, Qu Y, Zheng X (2014) Amplified fluorescence quenching of lucigenin self-assembled inside silica/chitosan nanoparticles by Cl−. Anal Chem 86:9114–9121
Kong R, Zhang X, Chen Z, Tan W (2011) Aptamer-assembled nanomaterials for biosensing and biomedical applications. Small 7:2428–2436
Ma D, Kell A, Tan S, Jakubek Z, Simard B (2009) Photophysical properties of dye-doped silica nanoparticles bearing different types of dye-silica interactions. J Phys Chem C 113:15974–15981
Huber C, Krause C, Werner T, Wolfbeis O (2003) Serum chloride optical sensors based on dynamic quenching of the fluorescence of photo-immobilized lucigenin. Microchim Acta 142:245–253
Xu Z, Huang X, Dong C, Ren J (2014) Fluorescence correlation spectroscopy of gold nanoparticles, and its application to an aptamer-based homogeneous thrombin assay. Microchim Acta 181:723–730
Zhang H, Shuang S, Sun L, Chen A, Qin Y, Dong C (2014) Label-free aptasensor for thrombin using a glassy carbon electrode modified with a graphene-porphyrin composite. Microchim Acta 181:189–196
Wang X, Zhao Q (2012) A fluorescent sandwich assay for thrombin using aptamer modified magnetic beads and quantum dots. Microchim Acta 178:349–355
Yue Q, Shen T, Wang L, Xu S, Li H, Xue Q, Zhang Y, Gu X, Zhang S, Liu J (2014) A convenient sandwich assay of thrombin in biological media using nanoparticle-enhanced fluorescence polarization. Biosens Bioelectron 55:231–236
Sinha B, Ramulu T, Kim K, Venu R, Lee J, Kim C (2014) Planar Hall magnetoresistive aptasensor for thrombin detection. Biosens Bioelectron 59:140–144
Cunningham J, Brenes N, Crooks R (2014) Paper electrochemical device for detection of DNA and thrombin by target-induced conformational switching. Anal Chem 86:6166–6170
Yue Q, Shen T, Wang L, Xu S, Li H, Xue Q, Zhang Y, Gu X, Zhang S, Liu J (2014) A convenient sandwich assay of thrombin in biological media using nanoparticle-enhanced fluorescence polarization. Biosens Bioelectron: 56231–56236
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant 21375085) and Innovation Funds for Graduate Programs of Shaanxi Normal University (No. 2011CXB009).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Ma, M., Zheng, X. Preparation of brightly fluorescent silica nanoparticles modified with lucigenin and chitosan, and their application to an aptamer-based sandwich assay for thrombin. Microchim Acta 182, 2193–2199 (2015). https://doi.org/10.1007/s00604-015-1554-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1554-0